Filing is a new policy for health food (dietary supplement) in China, which become effective in 2016. And since former Guangdong FDA issued the first domestic filed health food on 27th July, 2017, the number of approved filed products is growing rapidly in China. In order to help relevant enterprises have an overview of this kind of products, CIRS counted the data of the approved domestic and imported health food filing products from 2017 to 2021, and made an analysis for your reference.
1. The Number of Approved Filed Health Foods in China
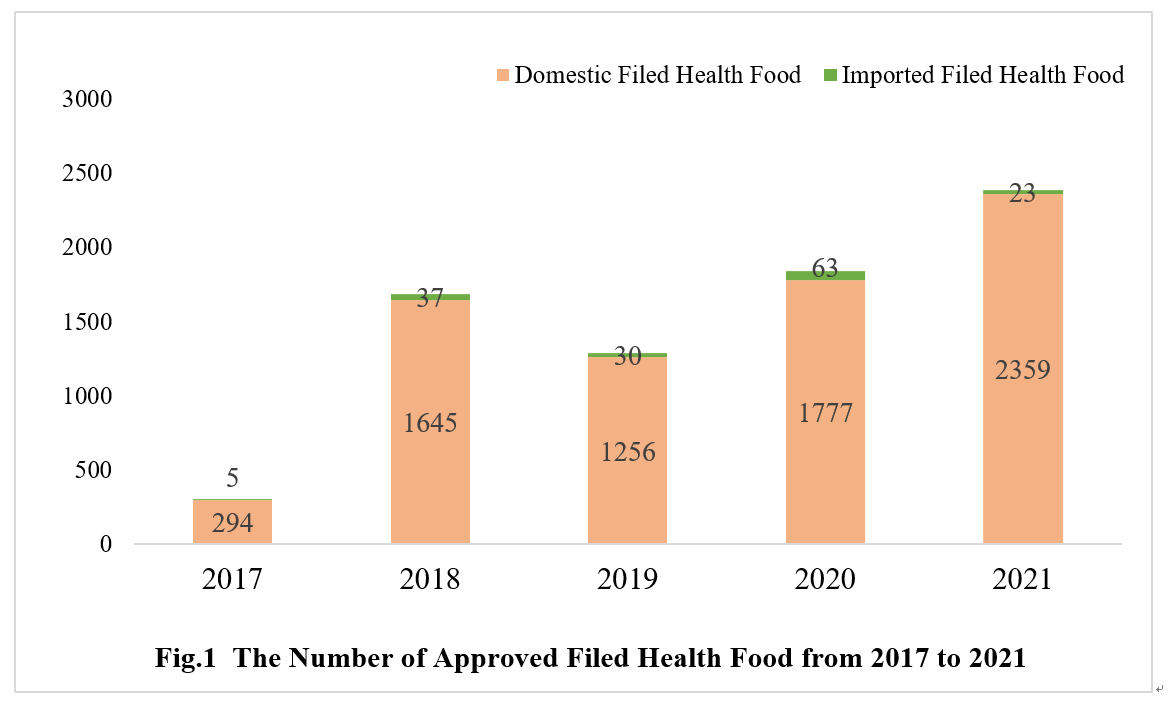
As of Dec. 31st 2021, the number of approved filed health foods in China has reached 7489. The quantity of domestic and imported filed health foods is 7331 and 158 respectively.
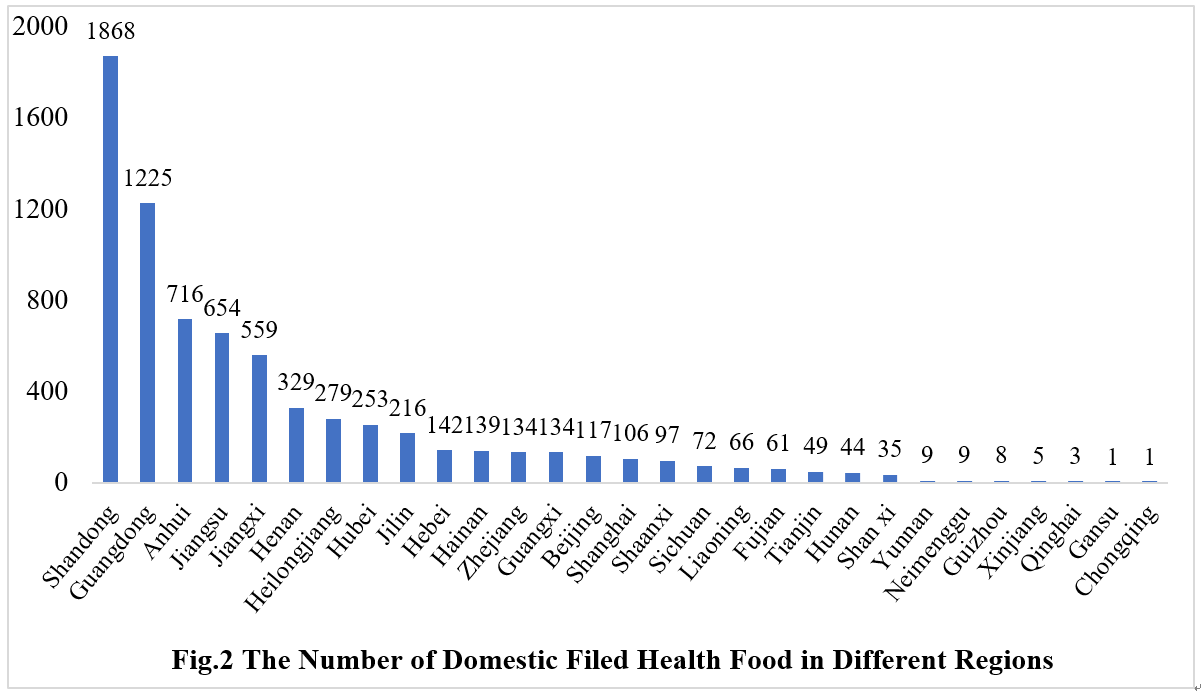
2. The Number of Approved Filed Health Foods in Different Regions
2.1 Domestic Filed Health Food
The number of approved domestic filed health foods is varied in different provinces in China. Shandong and Guangdong province rank the first and second place respectively with the number of 1868 and 1225, which account for 25.48% and 16.71% of total domestic products, respectively.
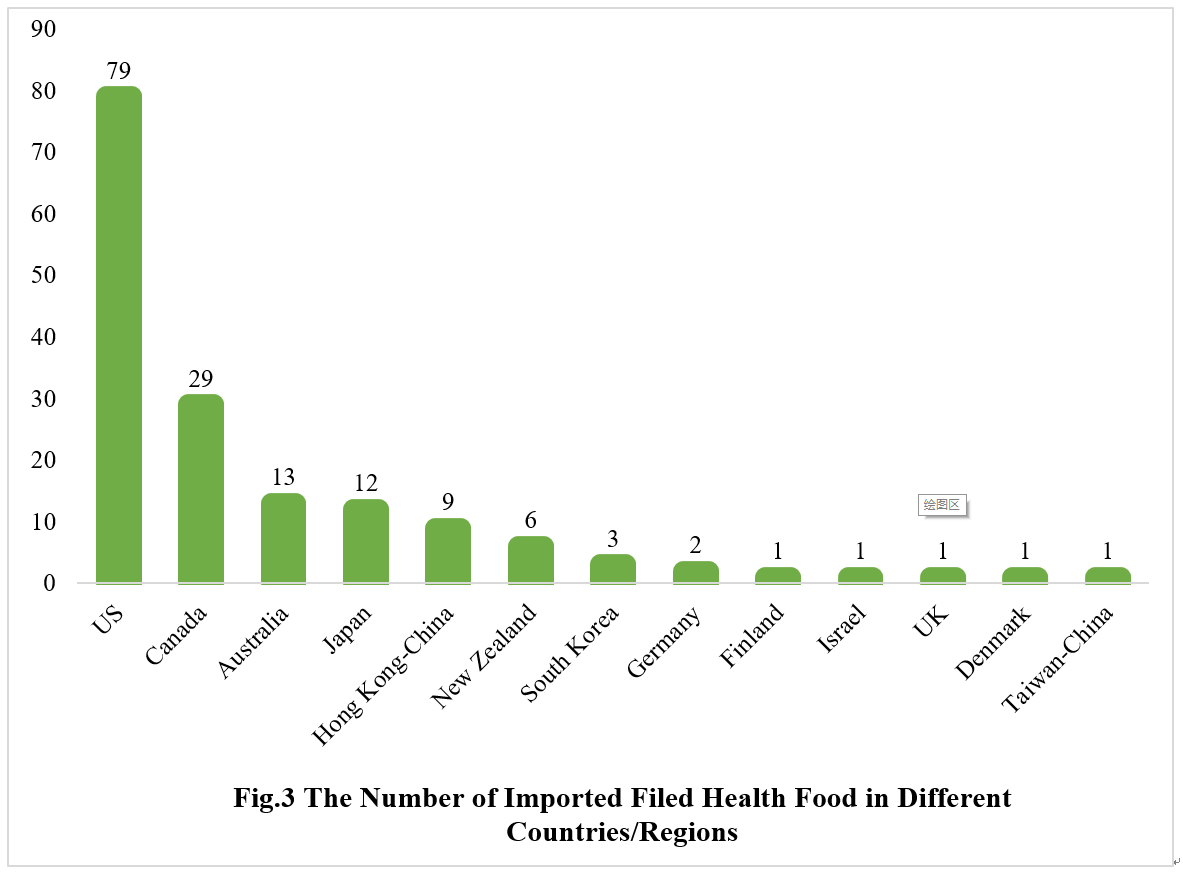
2.2 Imported Filed Health Food
The applicants come from the United States, Canada, Australia, Japan, Hong Kong-China, New Zealand, South Korea, Germany, Finland, Israel, the United Kingdom, Denmark and Taiwan-China have obtained filing certificates, with a total of 158 products filed, and 79 products are from American enterprises.
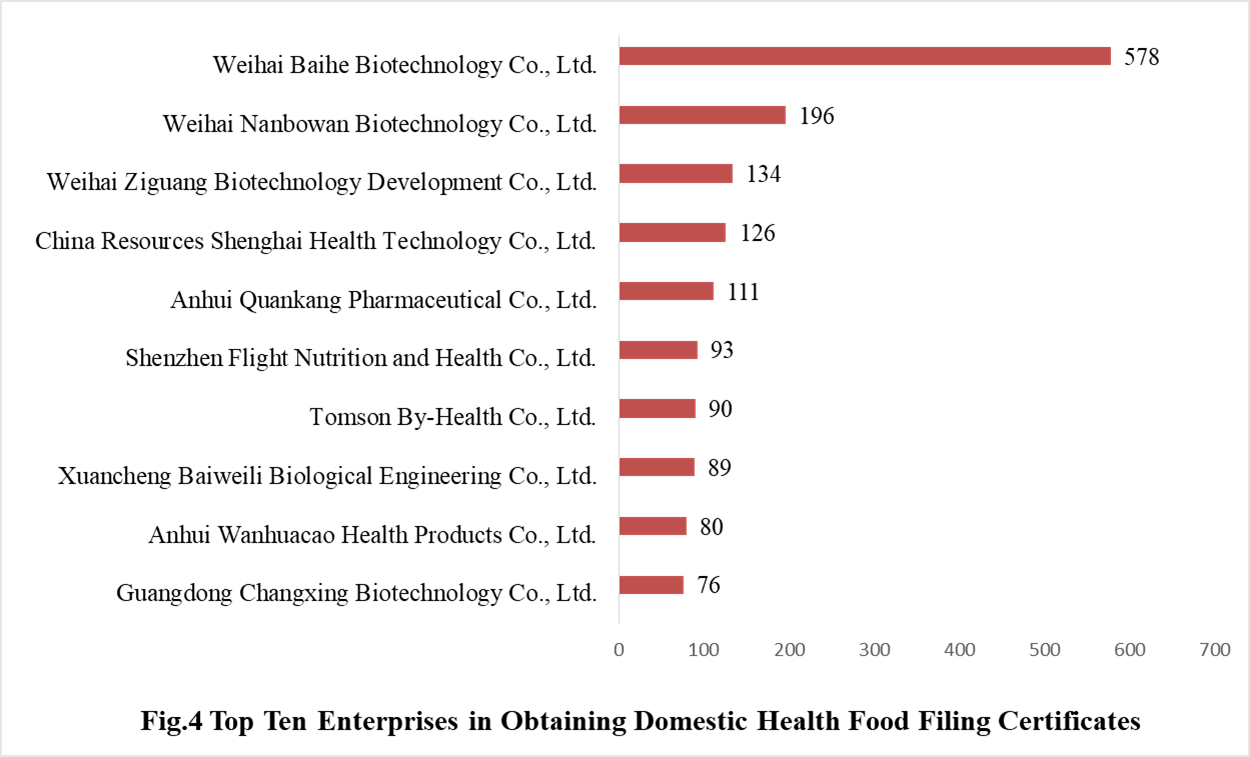
3. Enterprises Obtaining Health Food Filing Certificates
3.1 Domestic Filed Health Food
671 domestic health food manufacturers have obtained health food filing certificates. Among them, Weihai Baihe Biology Technology Co., Ltd. has got the largest number of filing certificates (578), followed by Weihai Nanbowan Biotechnology Co., Ltd. and Weihai Ziguang Biotechnology Development Co., Ltd. with the number reaching 196 and 134, respectively.
3.2 Imported Filed Health Food
35 oversea companies have got health food filing certificates, Jamieson Laboratories Ltd. has got the largest number of filing certificates (23). Mega Health International, Inc. ranks the second place with the number reaching 19, followed by USA BORNATOP INVESTMENT & MANAGEMENT CO., LTD, which numbered 11.
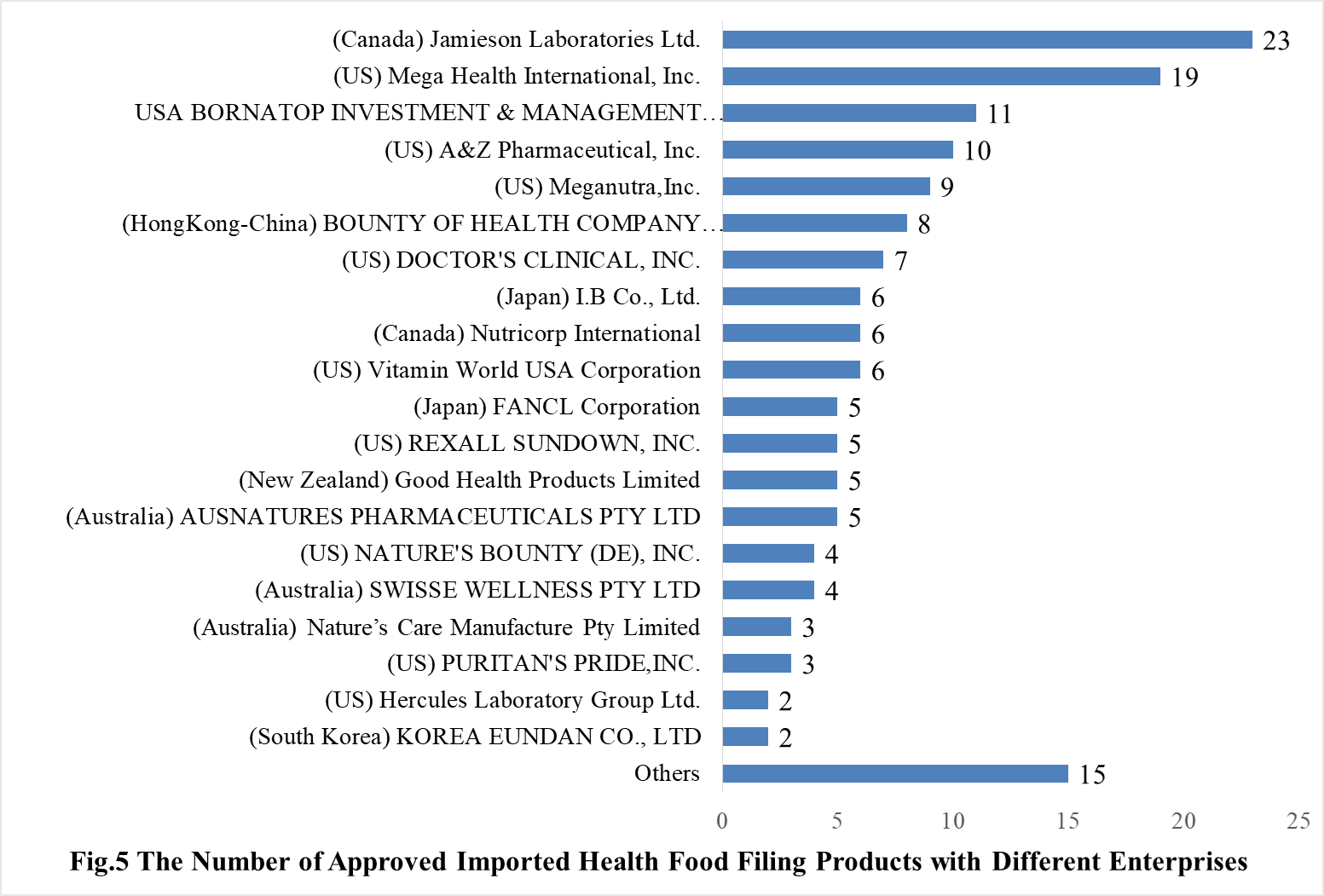
Note: Companies with 1 filed product are classified as “Other”.
4. The Number of Approved Health Food Filing with Different Dosage Forms
For the moment, 7 dosage forms in total are available for filing in China, namely, tablet, hard capsule, soft capsule, oral liquid (including drops), granule, gelatin candy and powder.
4.1 Domestic Filing Products
As can be seen from Fig 6, the most popular dosage forms of approved domestic filed health food are tablets, with the number of 4155, which account for 56.68% of total domestic products.
Powder and gelatin candy are the new dosage forms available for filing since July 1, 2021. As of December 31, 2021, 62 products in these 2 dosage forms have got the filing certificates, with 22 gelatin candy products and 40 powder products, respectively.
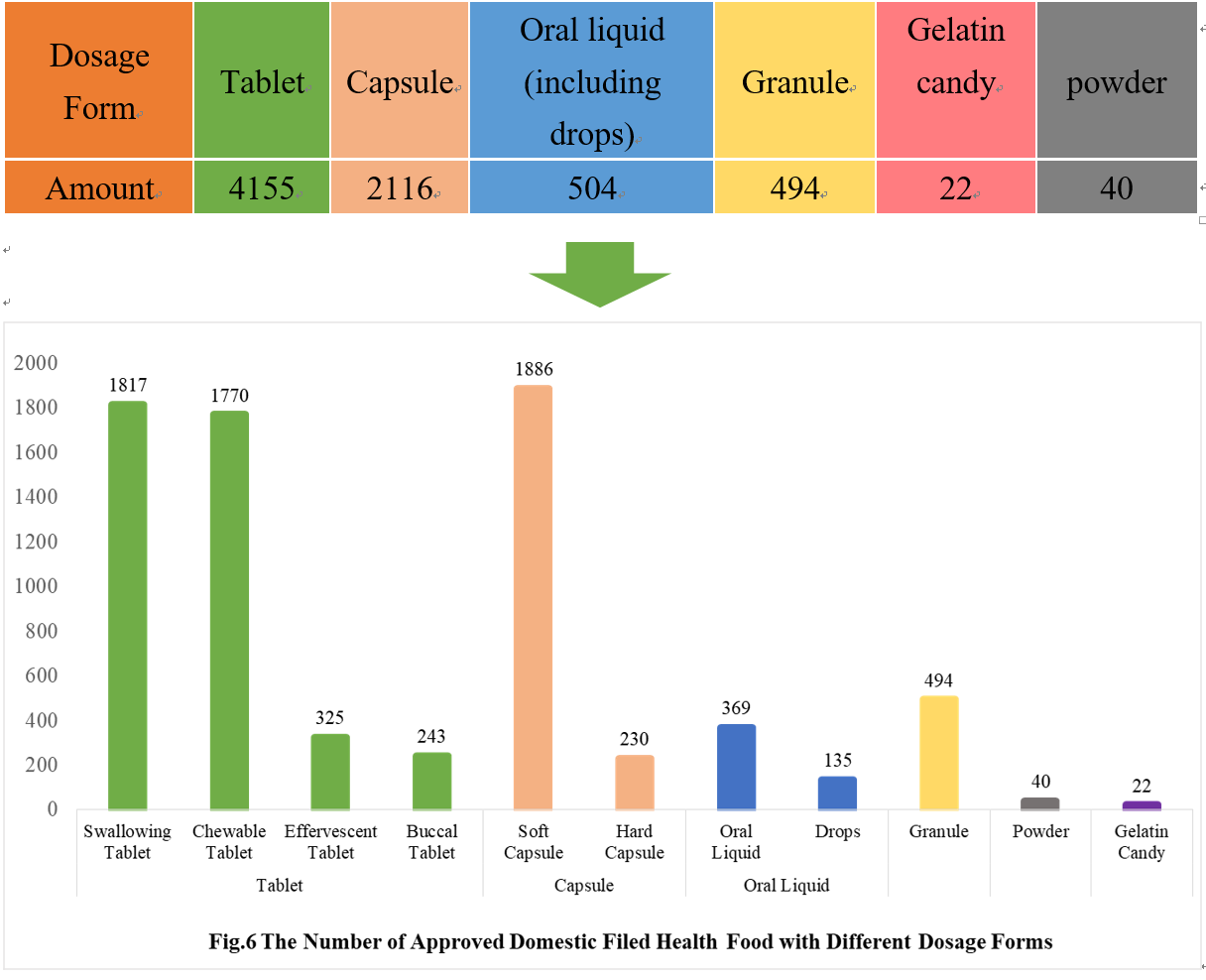
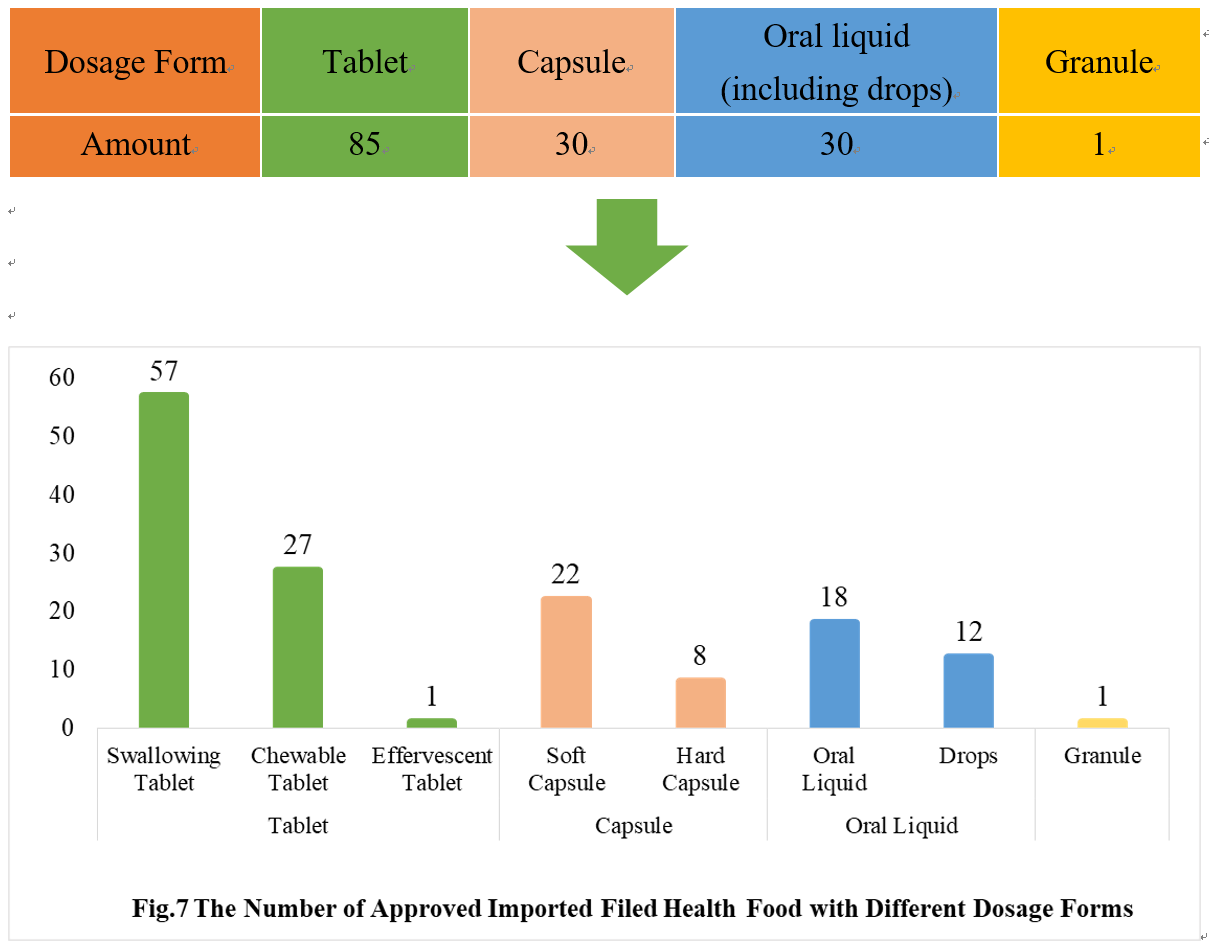
4.2 Imported Filing Products
Similar to the domestic product, the dosage forms of approved imported filed health food are mostly tablets as well, with the number of 85, which account for 53.80% of total imported products. No gelatin candy product or powder product has been filed among imported products.
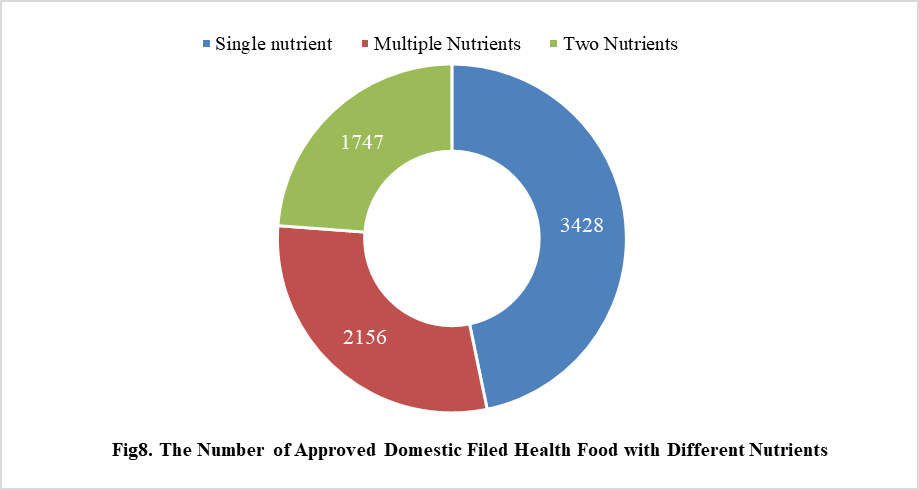
5. The Number of Approved Health Food Filing with Different Nutrients
5.1 Domestic Filed Health Food
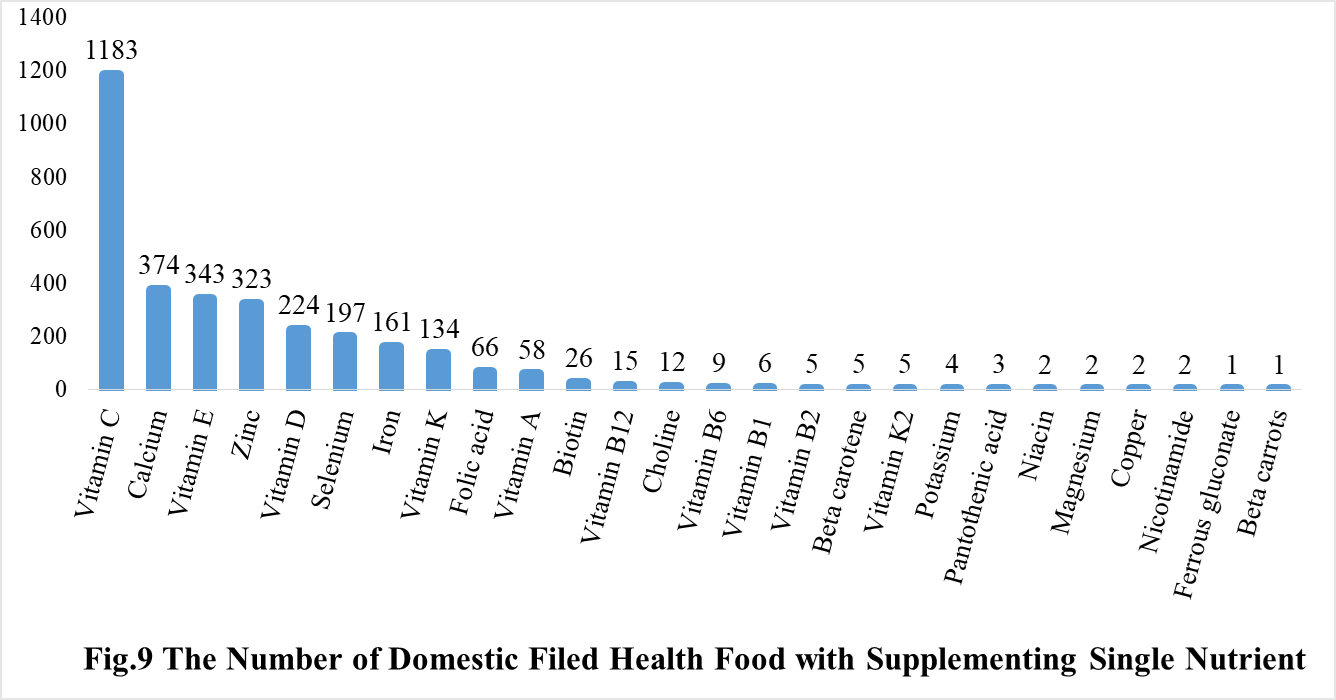
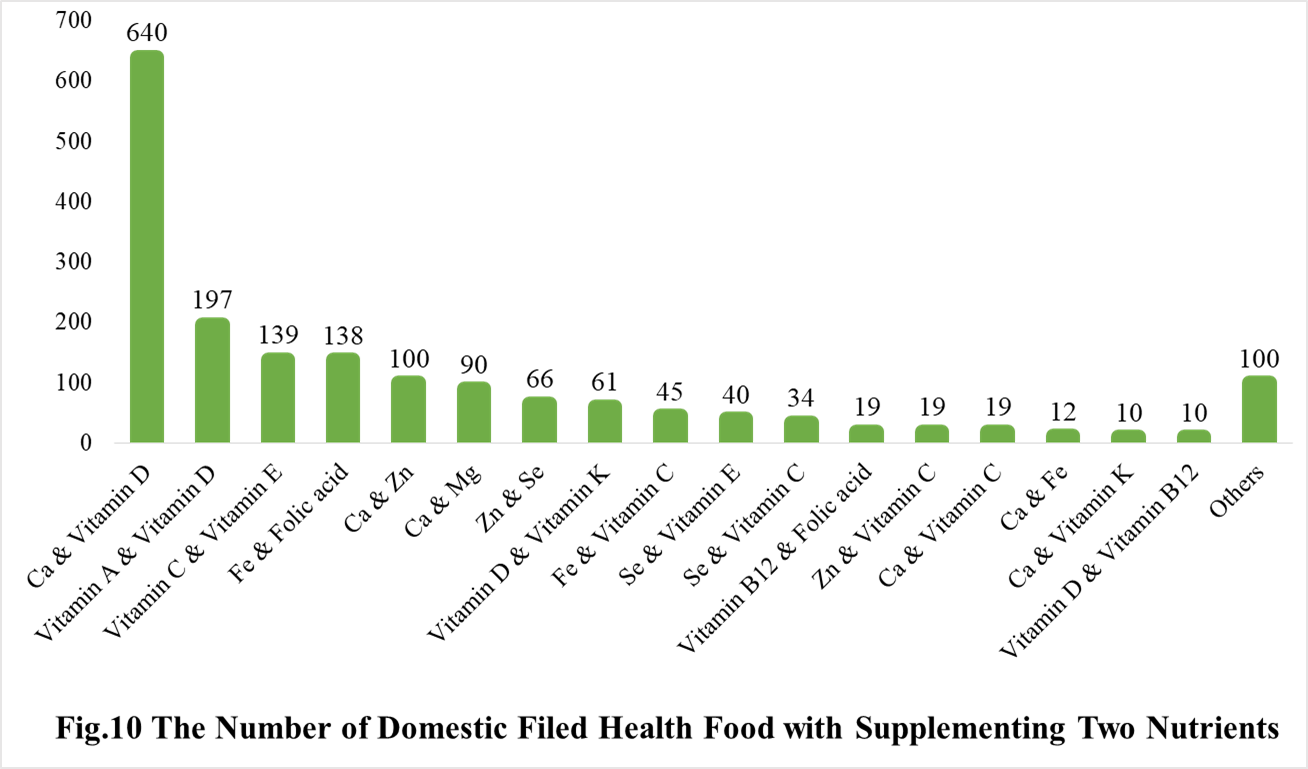
Among the domestic nutrition supplement products, the most popular products are multi-vitamins and minerals supplements, Vitamin C supplements, Calcium and Vitamin D supplements, which is 1286, 1183 and 640 respectively.
In addition, Ganoderma lucidum spore powder, melatonin, fish oil, coenzyme Q10 and spirulina products were transferred from registration to filing supervision for the first time in 2021, and the filing number is 76, 65, 61, 58 and 5 respectively.
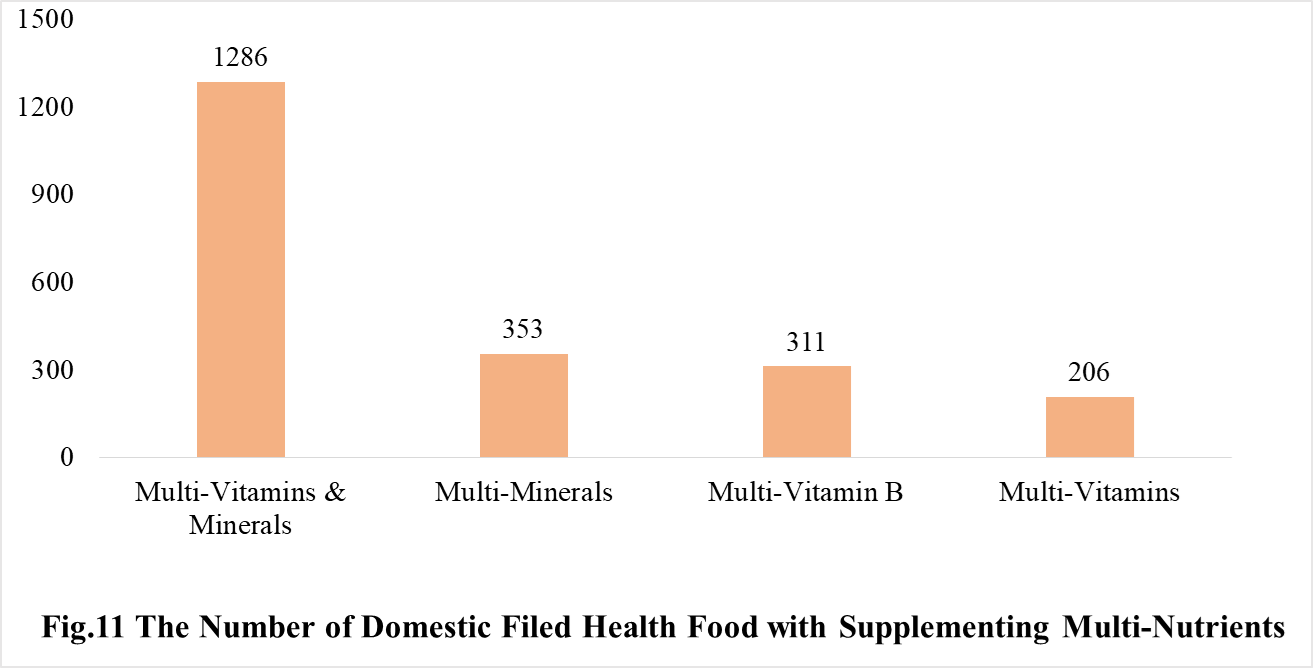
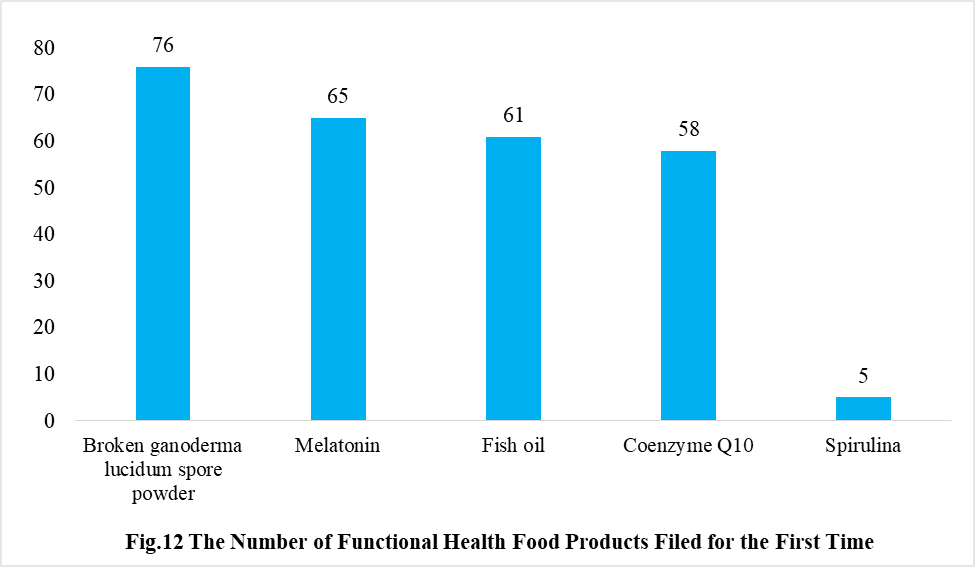
5.2 Imported Filed Health Food
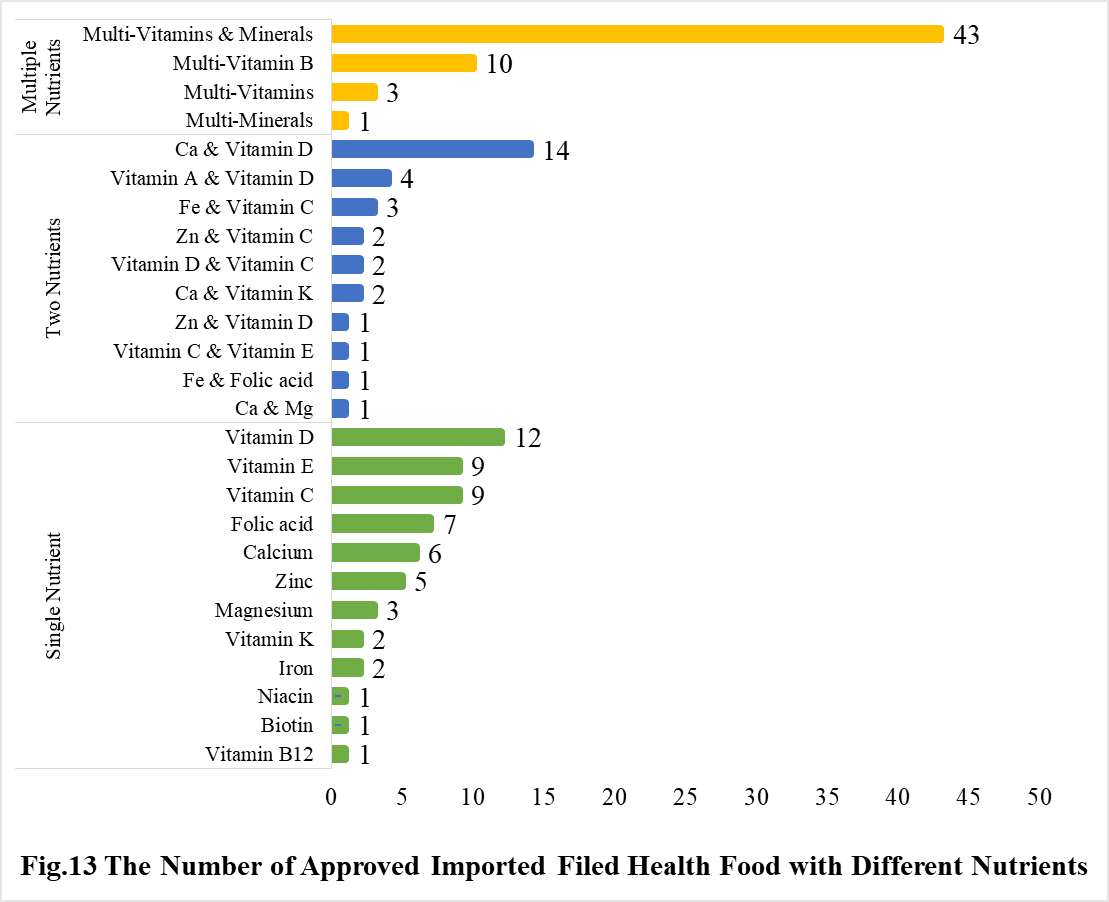
Among imported products, the most popular producnts are multi-vitamins and minerals supplements, Calcium and Vitamin D supplements, and Vitamin D supplements, which is 43, 14 and 12, respectively.
Note:
I. The data in this article is from the Special Food Information Query Platform, Center for Food Evaluation of SAMR, and Local Administration for Market Regulation.
II. There may be some omissions in the data of domestic filed health food due to the replacement of new and old websites of government departments after the reform of state institutions, thus the data in this article is for reference only, and please refer to the information published by the government.
III. The Special Food Information Query Platform and Center for Food Evaluation of SAMR lag behind in information release. According to CIRS’s knowledge, the information of some products that have obtained health food filing certificates have not been published on the official website of the government departments. Therefore, the actual record amount of health food exceeds the data listed in this article.
If you have any needs or questions, please contact us at service@cirs-group.com.

