Introduction of New Substance Notification under CEPA
New substance notifications are required under the Canadian Environmental Protection Act (CEPA). The Canadian chemical substance list includes two parts: the Domestic Substance List (DSL), which records more than 23,000 substances, and the Non-Domestic Substance List (NDSL) which records almost 58,000 substances.
The DSL is used to identify whether a substance is classified as a new substance in Canada. If the substance is not on the DSL, the substance is regarded as a new substance. Therefore, it shall be notified prior to manufacturing or importation. The NDSL determines the information to be provided for the registration. If a new substance is listed on the NDSL, the amount of data required for notification may be reduced.
NSNR is appropriate for:
- The domestic manufacturers in Canada.
- The importer within the borders of Canada.
NSNR is inappropriate for:
- The substance or polymer listed in DSL.
- The packaged substance transported via Canada.
- Substances regulated by other acts, such as the Pest Control Act, the Fertilizer Act, Feeds Act etc.
Notification Type
1. Chemicals and Biochemicals
If the substance is considered to be one of the following:
- a substance that is used for research and development.
- a substance that is a contained site-limited intermediate substance.
- A substance that is a contained ex-port-only substance.
Every legal entity that manufactures or imports a substance referred to in one of the above must provide the information specified in Schedule 1 (the information doesn’t refer to physicochemical, toxicity and eco-toxicity properties) to the Minister at least 30 days before the day on which the quantity of the substance exceeds 1000 kg in a calendar year. The detailed requirements are indicated in appendix Schedule 1.
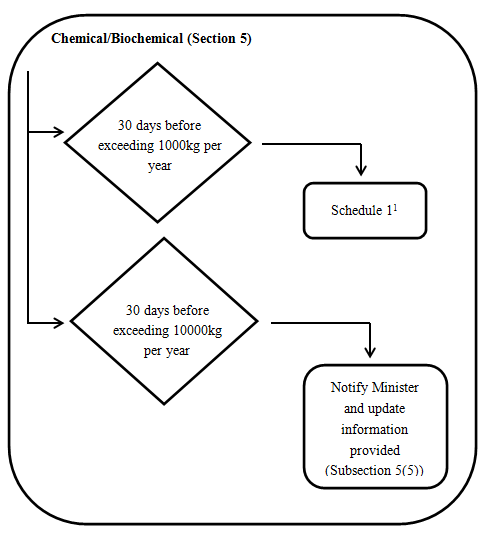
If the chemical is a biochemical research and development substance, the legal entity must provide, with the Schedule 1 information and the information specified in items 1 and 2 of Schedule 2.
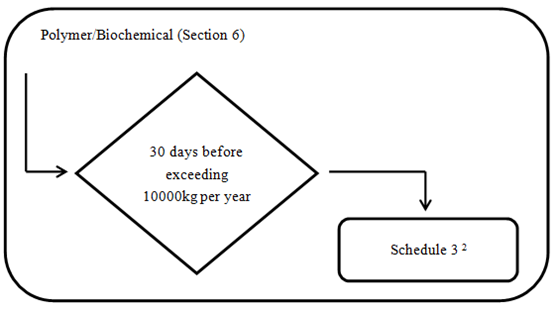
2. Polymers and Biopolymers
Every legal entity that manufactures or imports a polymer referred to in one of the following below must provide to the Minister the information specified in Schedule 3 at least 30 days before the day on which the quantity of the polymer exceeds 10 000 kg in a calendar year. The detailed requirements are indicated in appendix Schedule 3
- a polymer that is a research and development substance;
- a polymer that is a contained site limited intermediate substance; or
- a polymer that is a contained export only substance.
If the polymer is a biopolymer research and development substance, the entity must provide, within addition to the Schedule 3 information, the information specified in items 1 and 2 of Schedule 2.
3. Chemicals and Biochemicals on NDSL
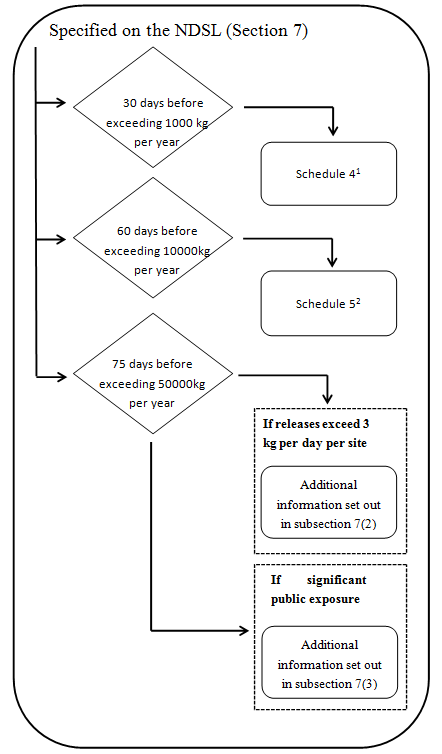
1 Additional information specified in Schedule 2 is also required if the substance is a biochemical- see subparagraph 7(1)(a)(ii).
2 Additional information specified in Schedule 2 is also required if the substance is a biochemical- see subparagraph 7(1)(a)(ii). No further information will be required unless:
(a) The substance is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment- see subsection 7(2).
or
(b) The public may be significantly exposed to the substance in a product – see subsection 7(3).
- Every legal entity that manufactures or imports a chemical that is on the NDSL must provide the following to the Minister at least 30 days before the day on which the quantity of the substance exceeds 1 000 kg in a calendar year.
- The information specified in Schedule 4.
- If the chemical is a biochemical, the information specified in items 1 to 3 of Schedule 2.
- Every legal entity that manufactures or imports a substance that is on the NDSL must provide the following to the Minister at least 60 days before the day on which the quantity of the substance exceeds 10 000 kg in a calendar year,
- The information specified in Schedule 5.
- If the substance is a biochemical, the information specified in items 1 to 4 of Schedule 2.
- If the quantity of the substance on NDSL exceeds 50 000 kg in a calendar year —and the chemical is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment. The legal entity must provide to the Minister the following information in respect of the substance, at least 75 days before the day on which the quantity exceeds 50 000 kg:
- The information specified in Schedule 5.
- For substances having a water solubility of greater than or equal to 200 μg/L, adsorption- desorption screening test data and the hydrolysis rate as a function of pH and, if known, the identity of the hydrolysis products.
- The data from a repeated-dose mammalian toxicity test of the substance for a duration of at least 28 days duration, using the most significant route of potential human exposure to the chemical, namely, oral, dermal or inhalation.
- If the quantity of the substance on NDSL exceeds 50 000 kg in a calendar year and if the public may be significantly exposed to the substance in a product, the legal entity must provide to the Minister the following information in respect of the substance, at least 75 days before the day on which the quantity exceeds 50000 kg:
- The information specified in Schedule 5.
- The data from a repeated-dose mammalian toxicity test of the substance for a duration of at least 28 days duration, using the most significant route of potential human exposure to the substance, namely, oral, dermal or inhalation.
- The data obtained from an in vitro test, with and without metabolic activation, for chromosomal aberrations in mammalian cells or the data from a previously existing in vivo mammalian test for chromosomal aberrations.
4. Chemicals and Biochemicals not on the NDSL
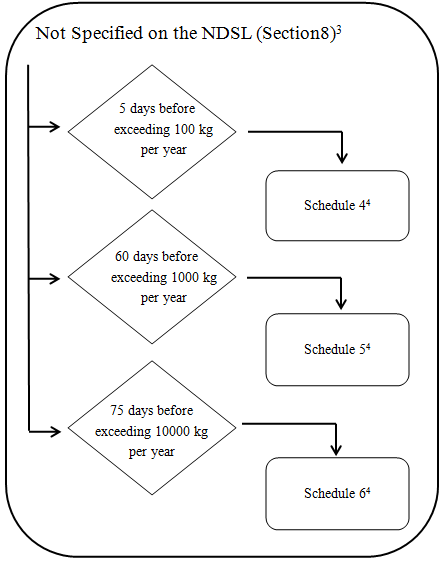
3 Notification must be sent to the Minister if: the chemical or biochemical is specified on the NDSL following submission of the information referred to in subparagraph 8(1)(b)(i) and item 10 of Schedule 5- see subsection 8(2).
4 Additional information specified in Schedule 2 is also required if the substance is a biochemical- see subparagraphs 8(1)(a)(ii), b(ii) and c(ii).
- Every legal entity that manufactures or imports a substance that is not on the NDSL must provide the following to the Minister at least 5 days before the day on which the quantity of chemical exceeds 100kg in a calendar year,
- The information specified in Schedule 4.
- If the substance is a biochemical, the information specified in items 1 to 3 of Schedule 2.
- Every legal entity that manufactures or imports a substance that is not on the NDSL must provide to the Minister at least 60 days before the day on which the quantity of chemical exceeds 1000kg in a calendar year,
- The information specified in Schedule 5, and
- If the substance is a biochemical, the information specified in items 1 to 4 of Schedule 2.
- Every legal entity that manufactures or imports a substance that is not on the NDSL must provide the following to the Minister at least 75 days before the day on which the quantity of chemical exceeds 10 000kg in a calendar year,
- The information specified in Schedule 6, and
- If the substance is a biochemical, the information specified in items 1 to 4 of Schedule 2.
5. Polymer Notification
Polymer notifications includes following types:
Reduced regulatory requirement polymers.
Polymers or biopolymers on the NDSL or all of whose reactants are on the DSL or NDSL.
Polymers and biopolymers not on the NDSL.
A reduced regulatory requirement polymer is:
- A polymer that is not one of the types listed in items 1 to 4 of Schedule 7 and that has an average molecular weight greater than 10 000 daltons, with less than 2% of its components having a molecular weight of less than 500 daltons and less than 5% of its components having a molecular weight of less than 1000 daltons.
- A polymer that is not one of the types listed in Schedule 7 and that has an average molecular weight greater than 1 000 daltons and equal to or less than 10 000 daltons, with less than 10% of its components having a molecular weight of less than 500 daltons and less than 25% of its components having a molecular weight of less than 1 000 daltons.
- A polymer that is a polyester manufactured solely from reactants listed in Schedule 8, or an anhydrous form of those reactants, other than the reactants or their anhydrous forms that include both 1-butanol and fumaric or maleic acid.
1) Reduced regulatory requirement polymers
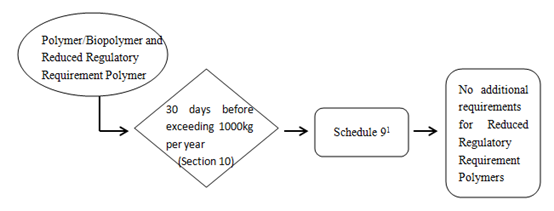
1 Required for polymers/biopolymers including reduced regulatory requirement polymers. Additional information specified in Schedule 2 is also required if the polymer is a biopolymer- see paragraph 10(b).
Every legal entity that manufactures or imports a polymer that must provide the following to the Minister at least 30 days before the day on which the quantity of polymer exceeds 1 000kg in a calendar year,
- The information specified in Schedule 9.
- Ff the substance is a polymer, the information specified in items 1 to 3 of Schedule 2.
2) Polymers and biopolymers on the NDSL or all of whole reactants are on the DSL or NDSL
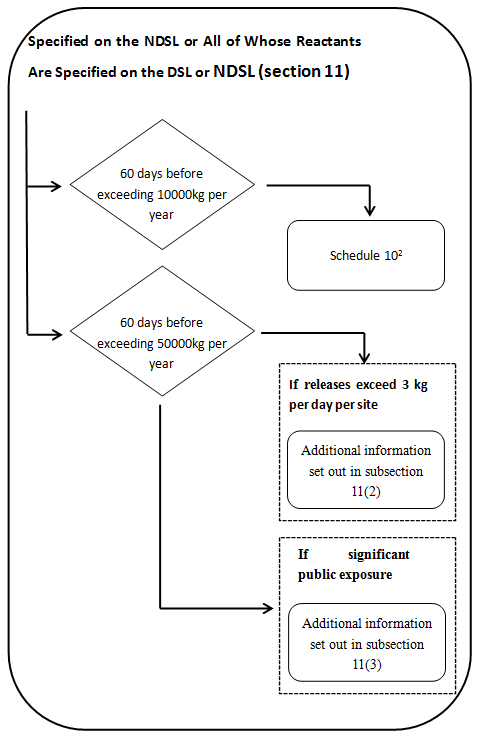
2 Not required for reduced regulatory requirement polymers. Also subject to certain exceptions- see subsection 11(5). Additional information specified in Schedule 2 is also required if the polymer is a biopolymer-see paragraph 11(1)(b). No further information will be required unless: (a) the polymer is released to the aquatic environmental in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment- see subsection 11(2)-or (b) the public may be significantly exposed to the polymer in a product –see subsection 11(3).
a) Every legal entity that manufactures or imports either a polymer that is on the NDSL or a polymer all of whose reactants are on the DSL or NDSL must provide the following to the Minister, at least 60 days before the day on which the quantity of the polymer exceeds 10 000 kg in a calendar year,
(i) The information specified in Schedule 10.
(ii) If the polymer is a biopolymer, the information specified in items 1 to 4 of Schedule 2,
(iii) If the biopolymer is a nucleic acid, the information specified in items 5 and 6 of that Schedule.
b) If the quantity of the polymer exceeds 50 000 kg in a calendar year —and the polymer is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment—the legal entity must, in addition to the information specified in Schedule 10, provide to the Minister the following information in respect of the polymer, at least 60 days before the day on which the quantity exceeds 50 000 kg:
(i) The data from a repeated-dose mammalian toxicity test of the polymer for a duration of at least 28 days duration, using the most significant route of potential human exposure to the polymer, namely, oral, dermal or inhalation.
(ii) The mutagenicity data obtained from an in vitro test, with and without metabolic activation, for gene mutation or chromosomal aberrations in mammalian cells.
Acute toxicity testing data of Schedule 10 and the above information are not required if the polymer does not meet the criteria for a reduced regulatory requirement polymer solely owing to the presence of any of the following functional groups:
(i) Aldehydes whose functional group equivalent weight is less than or equal to 1 000 daltons.
(ii) Vinyl ethers whose functional group equivalent weight is less than or equal to 5 000 daltons.
(iii) Sulphonic acids whose functional group equivalent weight is less than or equal to 5 000 daltons.
c) If the quantity of the polymer exceeds 50 000 kg in a calendar year and if the public may be significantly exposed to the polymer in a product, the legal entity must, in addition to the information specified in Schedule 10, provide to the Minister the following information in respect of the polymer, at least 60 days before the day on which the quantity exceeds 50 000 kg:
(i) The data from a repeated-dose mammalian toxicity test of the polymer for a duration of at least 28 days duration, using the most significant route of potential human exposure to the polymer, namely, oral, dermal or inhalation.
(ii) The mutagenicity data obtained from an in vitro test, with and without metabolic activation, for gene mutation.
(iii) The data obtained from an in vitro test, with and without metabolic activation, for chromosomal aberrations in mammalian cells or the data from a previously existing in vivo mammalian test for chromosomal aberrations that, together with data substantiating that the tissue investigated was exposed to the polymer or its metabolites, permits an assessment of in vivo clastogenicity.
Acute toxicity testing data of Schedule 10 and the above information are not required if the polymer does not meet the criteria for a reduced regulatory requirement polymer solely owing to the presence of any of the following functional groups:
(i) Aldehydes whose functional group equivalent weight is less than or equal to 1 000 daltons.
(ii) Vinyl ethers whose functional group equivalent weight is less than or equal to 5 000 daltons.
(iii) Sulphonic acids whose functional group equivalent weight is less than or equal to 5 000 daltons.
3) Polymers and biopolymers not on the NDSL
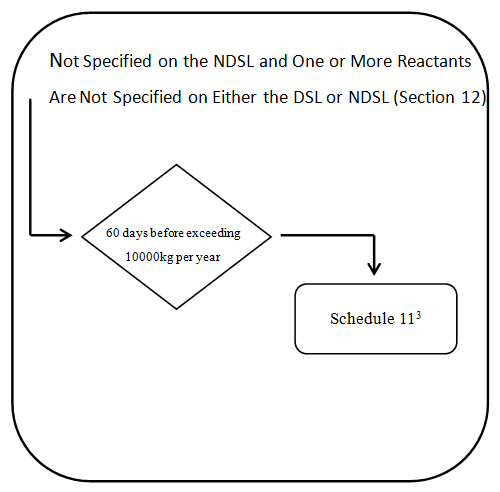
3 Not required for reduced regulatory requirement polymers. Also subject to certain exceptions- see subsection 12(3). Additional information specified in Schedule 2 is also required if the polymer is a biopolymer-see paragraph 12(1)(b).
Every legal entity that manufactures or imports a polymer that is not on the NDSL must provide to the Minister, at least 60 days before the day on which the quantity of the polymer exceeds 10 000 kg in a calendar year,
(i) The information specified in Schedule 11.
(ii) If the polymer is a biopolymer, the information specified in items 1 to 4 of Schedule 2.
(iii) If the biopolymer is a nucleic acid, the information specified in items 5 and 6 of that Schedule.
The acute mammalian toxicity testing data, skin irritation testing data, skin sensitization testing data, repeated-dose mammalian toxicity testing data for a duration of at least 28 days duration and mutagenicity data of Schedule 11 are not required if the polymer does not meet the criteria for a reduced regulatory requirement polymer solely owing to the presence of any of the following functional groups:
(i) Aldehydes whose functional group equivalent weight is less than or equal to 1 000 daltons.
(ii) Vinyl ethers whose functional group equivalent weight is less than or equal to 5 000 daltons.
(iii) Sulphonic acids whose functional group equivalent weight is less than or equal to 5 000 daltons.
Our Services:
- NDSL and DSL inquiry;
- GLP testing monitoring;
- New substance notification in Canada.