Taiwan's industrial chemicals are currently managed by the Toxic and Concerned Chemical Substance Control Act (TCCSCA) and the Occupational Safety and Health Act (OSHA). Under TCCSCA, enterprises manufacturing or importing new substances or a given quantity of existing chemical substances shall register those substances with the Environmental Protection Administration (EPA).
On November 21, 2021, EPA released the latest revised edition of the Regulation on New and Existing Chemical Substances Registration. This regulation, known as the subordinate regulation of TCCSCA, has set out detailed information requirements for chemical registrations under TCCSCA.
Who Shall Register
- Manufacturers of chemical substances in Taiwan;
- Importers of chemical substances in Taiwan;
The Registrants must be the legal entities or natural person in Taiwan. Registrants may entrust a third party representative (TPR) to complete registration, and representatives must be the legal entities or natural person in Taiwan.
Definition and Scope of Exemption
Existing Chemical Substances: Substances that are listed in Taiwan Chemical Substance Inventory (TCSI);
New Chemical Substances: Substances that are not listed in Taiwan Chemical Substance Inventory (TCSI);
Scope of Exemption:
- Naturally occurring substances;
- Chemical substances accompanied in the machines and equipment for test-run purpose;
- Non-isolated intermediates from chemical reaction in the reaction vessel or in the production process;
- Chemical substances for security or defense;
- Chemical substances under customs supervision;
- Chemical wastes produced or released from industrial process;
- By-products or impurities not for commercial purpose;
- Mixtures (not applicable to the individual chemical constituents of the mixture);
- Articles;
- Polymers listed on the TCSI (Taiwan Chemical Substance Inventory) and subject to the 2% rule;
- Substances controlled by other regulations.
Taiwan Chemical Substance Inventory (TCSI)
Substances not listed in Taiwan Chemical Substance Inventory (TCSI) are regarded as new chemical substances subject to new chemical substance registration. Currently, TCSI contains over 100,000 chemical substances. New chemical substances that have completed standard registration in Taiwan and meet the required conditions will also be supplemented into TCSI.
The Taiwan Chemical Substance Inventory (TCSI) can be searched here.
New Chemical Substance Registration
Based on the tonnage band, substance properties and usage information, three registration types will be adopted, including standard registration, simplified registration, and small quantity registration.
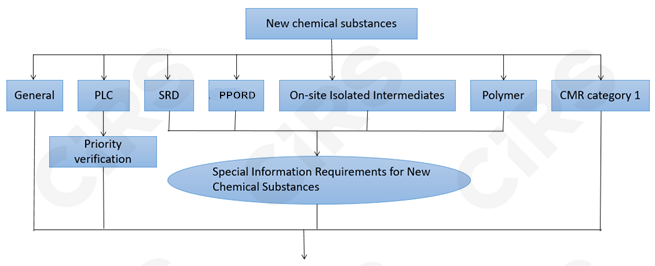
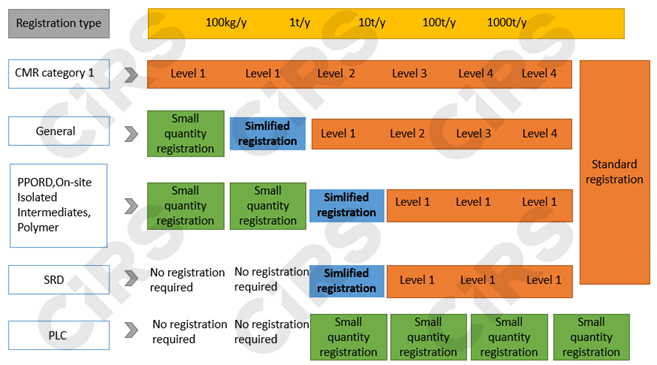
Registration Materials
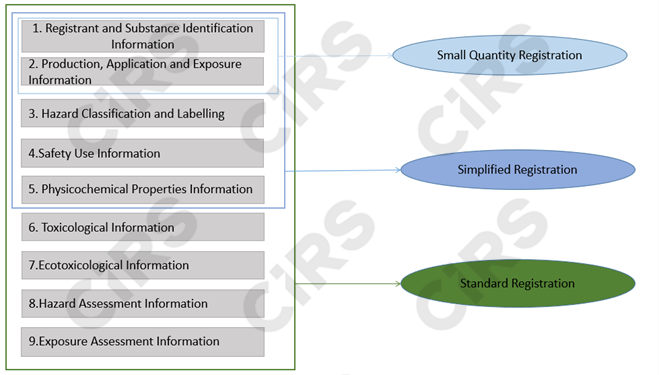
Note: (1) Chemical substances falling under special circumstances must submit additional documents for those special cases; (2) For standard registration, hazard assessment information and/or exposure assessment information shall be submitted based on the substance's hazard characteristics and the tonnage band.
Joint Registration
When different registrants apply to register the same new chemical substance jointly or sequentially, they may share common data for the registration through consultation.
The new chemical substance, subject to the joint registration, is to be registered according to the Regulations, for which the overall quantity of the joint registration shall be the sum of the individual quantities from each co-registrant.
By taking into account the overall manufactured or imported quantity of the new chemical substances registered and approved, the central competent authority may require registrants to apply for the new registration under the designated registration type or to apply for joint registration.
Review Period and Validity Period
The Review Period for different types of registration are as follows:
- 7 working days for new chemical substances with small quantity, PLC prior verification, PLC registration with small quantity and CBI protection;
- 14 working days for simplified registration or inclusion to the TCSI;
- 45 working days for standard registration.
Competent authority shall notify the registrants if the review periods to be extended, up to 1. If additional data is requested or corrected, the review period will restart.
Corrections should be completed within 30 working days from the date of receipt of the correction notice. The number of corrections are limited to twice. If the application still does not pass the review after two corrections, it will be rejected. If corrections cannot be completed on time due to scientific or technical reasons, an application shall be made to the competent authority, and the correction time will not be included in the review time.
The confidential period of the information on chemical substances approved by the central competent authority is valid for 5 years. A registrant may apply for extension of a confidential period three to six months prior to expiry of the period. The maximum confidential period for a new chemical substance is 15 years. For a new chemical substance that has been included in the TCSI, the maximum confidential period is 15 years.
The validity period of the information on chemical substances approved by the central competent authority is 5 years. For joint registration sequentially, the validity period of the later applicant's registration is the same as the approved registration validity period of the earlier applicant.
A registrant may apply for extension of a validity period three to six months prior to expiry of the period.
Review Fee
Registration Type | The Review Fee for Registration Information (NTD) | The Review Fee for Extension of Registration Materials (NTD) |
Standard Registration | 50,000 | 2,000 |
Simplified Registration | 20,000 | 1,000 |
Small Quantity Registration | 2,000 | 1,000 |
PLC Review | 1,000 | |
Confidentiality of Information | 12,500/Item | |
Confidentiality Extension of Information | 10,000/Item |
Note:
(1) If the registrant belongs to an academic institution or a small or medium-sized enterprise, the standard registration, simplified registration, and small quantity registration are charged at 75% of the review fee.
(2) For standard registration, if data on skin irritation/corrosion or eye irritation is submitted in the form of in vitro tests, QSAR reports, or read-across data, the review fee is 37,000 NTD.
(3) Items eligible for confidentiality include registrant information, chemical identification information, production or importation information and application information.
(4) Reference: Fee-charging Standards for Toxic and Concerned Chemical Substances Control Act (2021).
Existing Chemical Substance Registration
The registration types for existing chemical substances are mainly divided into Phase 1 registration and Standard registration. Details of the registration type are specified in the following chart:
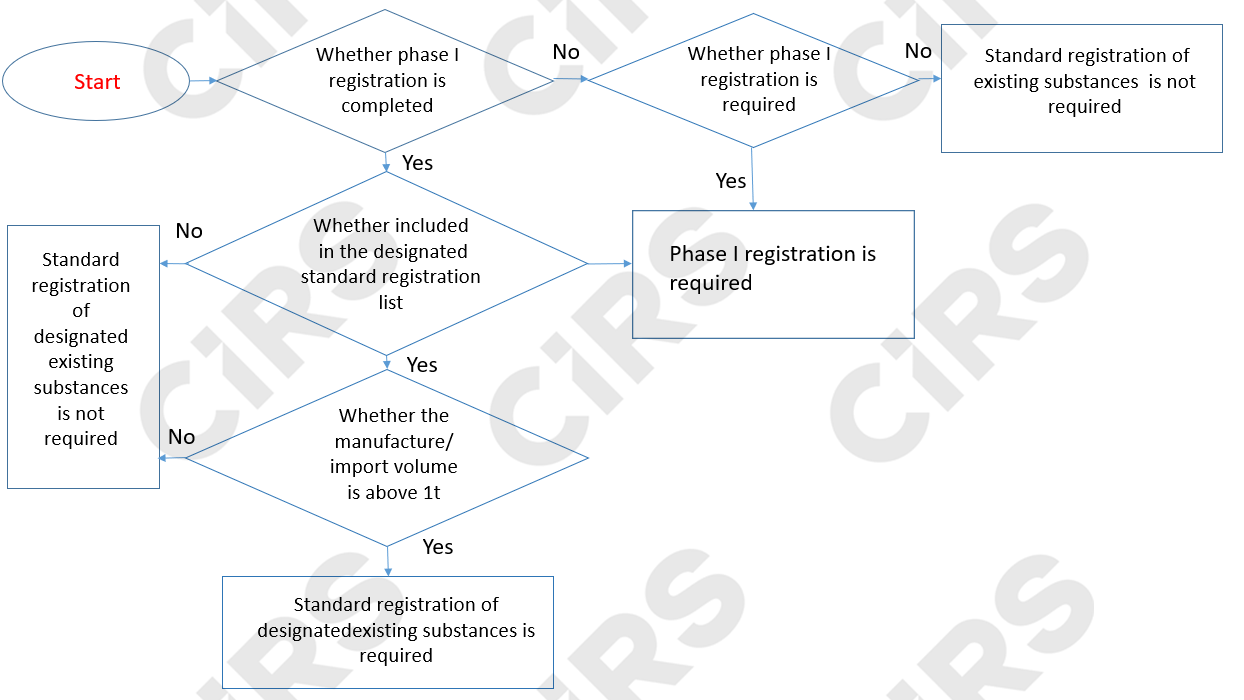
Existing Chemical Substance Phase I Registration
Registrants are required to submit Phase 1 registration documents to competent authorities prior to manufacture or import if the annual volume of manufactured or imported existing chemicals above 100kg.
Data Requirements for Phase I Registration | |
1. Registrant and substance identification | 1.1 Registrant information 1.2 CAS No. or serial number |
2. Substance production and application | 2.1 Production and import volume 2.2 Intended uses 2.3 Production/import activity certificate |
Standard Registration
The competent authority may, by stages, designate the lists of existing chemical substances subject to standard registration, including the names of chemical substances, quantity thresholds and deadlines for registration, based on the circumstances of the phase 1 registration of existing chemical substances.
The list of the first batch of 106 Designated Chemical Substances subject to standard registration was released in March 2019. The Data requirement for existing chemicals standard registration is the same as standard registration of new substances. For the substances that obtained the phase I registration number before Dec 31, 2019, the company should finish standard registration before the deadlines and will be forbidden to manufacture or import these 106 substances into Taiwan after the deadline, unless they finish the registration. The deadlines for standard registration are as follows:
Situation of Phase I Registration | Deadline for Standard Registration | |
Obtain Phase I Registration code before Dec. 31 2019 | Annual quantity ≥1 ton | Before Dec 31, 2024 |
Obtain Phase I Registration code after Jan. 1 2020 | Annual quantity ≥1 ton | Registration must be completed within 5 years from Jan 1 of the following year. |
The annual tonnage is less than 1 ton when the Phase I Registration code is obtained for the first time | The actual annual volume≥1 ton before Dec. 31, 2019 | Before Dec 31, 2024 |
The actual annual quantity≥1 ton after Jan 1, 2020. | Registration must be completed within 5 years from Jan 1 of the following year. | |
Note: If the applicant submitted a new application for Phase I registration after the original registration code is withdrawn, the standard registration shall still be completed within the previous deadline upon obtaining the registration code. If the new application is made after the designated deadline, the standard registration shall be completed simultaneously with the application.
Volume threshold
Annual quantity of chemicals manufactured or imported | CMR Category 1 Substance | Other Chemical Substances |
1 ton ≤Q<10 ton | Level 2 | Level 1 |
10 ton ≤Q<100 ton | Level 3 | Level 2 |
100 ton ≤Q<1000 ton | Level 4 | Level 3 |
1000 ton ≤Q | Level 4 | Level 4 |
Note: After standard registration, if the actual annual quantity manufactured or imported increases, resulting in a change in quantity thresholds, the registrant shall submit additional documents to competent authorities according to the new quantity thresholds.
Data Requirements
Data | Level 1 | Level 2 | Level 3 | Level 4 |
Registrant and substance identification | √ | √ | √ | √ |
Production, application and exposure information | √ | √ | √ | √ |
Hazard classification and labeling | √ | √ | √ | √ |
Safe use | √ | √ | √ | √ |
Physical and chemical properties | √ | √ | √ | √ |
Toxicological information | √ | √ | √ | √ |
Eco-toxicological information | √ | √ | √ | √ |
Hazard assessment | √ | √ | √ | |
Exposure assessment | √ | √ | √ |
Joint registration
When different registrants apply to register the same existing chemical substance jointly or sequentially, they may use common data in item 3, 5, 6, 7 and 8 for the registration.
After completing the registration, LR can provide a joint registration authorization code to other registrants. Other joint registrants only need to provide items 1, 2, 4, and 9 of the data requirements. With the joint registration authorization code, they can complete the registration data items.
Review period and validity period
The review period for the phase 1 registration of existing chemical substances is 7 working days, while the review period for the standard registration of existing chemical substances is 90 working days. The competent authority may extend the review period once and notify the registrant. If the registrant makes corrections based on the comments, the review time will be recalculated.
Upon receiving the correction notice, the registrant shall complete the correction within 30 working days, with a limit of two corrections. If the application is still not approved after two corrections, it will be rejected by the competent authority. If the registrant cannot complete the correction on time due to scientific/technical reasons, they may apply for an extension of the correction period from the competent authority.
After obtaining approval for Confidential Business Information (CBI) protection for existing chemical substances, the confidentiality period is 5 years. Six months before the expiration of the CBI protection period, the registrant may apply for an extension within 3 months. The cumulative maximum confidentiality period is 10 years.
Review Fee
Registration Type | Review Fee for Registration (NTD) |
Phase I Registration | 100 /Substance |
Standard Registration | 50,000 |
Confidentiality of Information | 12,500/Item |
Confidentiality Extension | 10,000/Item |
Note: If the registrant is from an academic institution or a small or medium-sized enterprise, the standard registration fee is charged at 75% of the review fee. For standard registration, if skin irritation/corrosion or eye irritation data is submitted in the form of in vitro tests, QSAR reports, or Read Across data, the official review fee is 37,000 NTD.
Annual Declaration
According to Article 24 of the Regulations of New and Existing Chemical Substances Registration, for new and existing chemical substances with registration approval, the registrant shall declare the quantity of substances manufactured and imported in the previous year from Apr 1 to Sep 30.
Data Requirements for Annual Declaration | |
1. Registrant and registration code | 1.1 Registrant information 1.2 registration approval code |
2. Substance manufacture/import quantity | 2.1 Quantity of chemicals manufactured last year 2.2 Quantity of chemicals imported last year |
Our Service
General consulting & Training
Search and confirm if a substance is listed in TSCI
Existing substance Pre-registration (Phase I registration)
Existing substance standard registration
Local Third Party Representative Service
New substance notification (Small volume, Simplified, and Standard)
Test monitoring/translation of study reports
Preparation and Submission of chemical safety report
Preparation of SDS and label in compliance with Taiwan GHS
Regulatory update monitoring
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.