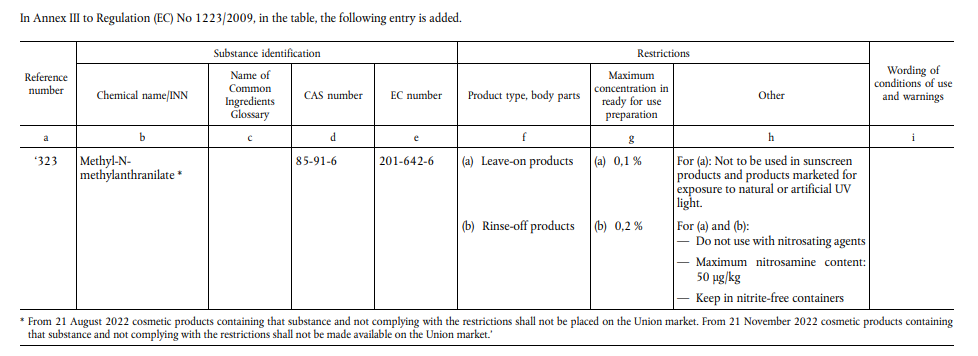
On 1 February 2022, EU has published official Journal (EU) 2022/135 amending Appendix III of (EC) No 1223/2009 by adding article 323 to clarify the restrictions on the use of Methyl-N-Methylanthranilate.
The article will come into force on 21 February 2022, and cosmetics that do not meet the requirements of the new article will not be placed on the EU market from 21 August 2022 and will not be made in EU from 21 November 2022.

Methyl-N-methylanthranilate(M-N-MA)(CAS no 85-91-6) is a fragrance ingredient used in a variety of cosmetics, including perfumes, shampoos and other cosmetics.
SCCS concluded in an opinion adopted at its plenary meeting of 13-14 December 2011 (SCCS/1455/11) that there were no safety concerns for the use of M-N-MA in a concentrations up to 0.2% in rinsing-off products. It further noted that M-N-MA is phototoxic although M-N-MA is safe at 0.1% in most leave-on cosmetics. However, safety risks cannot be ruled out for its use in sun protection products, sun care products or products (including perfumes) where the body is exposed to light.
In the plenary meeting on 27 March 2012, SCCS adopted an opinion on nitrosamines and secondary amines (SCCS/1458/11). In this opinion, SCCS concluded that secondary amines should not be used with nitrosating agent and the nitrosamine content of the ingredient should be less than 50μg/kg, and should not be in contact with nitrite treated raw material containers. This opinion applies to M-N-MA, which is a secondary amine.
On 16 October 2020, SCCS opinion on M-N-MA (SCCS/1616/20) stated that M-N-MA should not be used in sunscreen products and products exposed to natural or artificial UV light. For other cosmetics, SCCS considered the use of M-N-MA safe in a concentration of up to 0.1% for leave-on products and 0.2% for rinse-off products.
Based on the above comments and suggestions, EU decided to include M-N-MA as a restricted substance of cosmetics for supervision. Article 323 of Appendix III of the EU Cosmetics Regulation is newly added and will come into force from 21 February 2022. Cosmetics that do not meet the requirements of the new regulation cannot be placed on the EU market from 21 August 2022 and cannot be made in EU from 21 November 2022. The specific restriction conditions of M-N-MA are as follows:
- The maximum use concentration is 0.1% for leave-on products and 0.2% for rinse-off products.
- Do not use in sunscreen products and products exposed to natural or artificial UV light.
- Do not use with nitrosating agents. The maximum content of nitrosamine is 50μg/kg. Store in nitrite-free container.
M-N-MA has not been listed in the IECIC yet.
Reference:
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R0135

