According to the updates on the Union List of Novel Foods, CIRS has conducted a brief statistical comparison of the approval status of Novel Foods in the past three years (see the chart below).
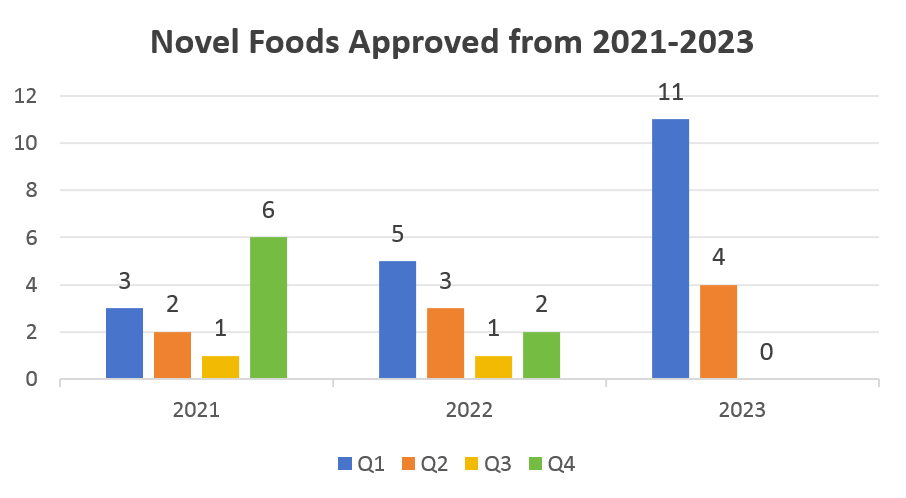
It is shown that the European Union (EU) has maintained a relatively stable trend of substance approvals in Q2 over recent years, while Q1 and Q4 experience notable fluctuations. In contrast, Q3 has seen only one approval in 2021 and 2022, and none this year.
Turning our focus to Q4, two new HMO substances, 3-FL and 6'-SL, have been approved, both of which are of microbial origin.
Related articles
Q1 2023 Novel Food Approval Summary
Q2 2023 Novel Food Approval Summary
3-Fucosyllactose produced by a derivative strain of Escherichia coli K-12 DH1

On October 23, 2023, the European Commission issued the COMMISSION IMPLEMENTING REGULATION (EU) 2023/2210, and authorizing the placing on the market of 3-Fucosyllactose produced by a derivative strain of Escherichia coli K-12 DH1 as a novel food and amending Implementing Regulation (EU) 2017/2470. This Regulation entered into force on November 12, 2023.
Applicant: Glycom A/S
By the end of November 12, 2028 (data protection period), the novel food 3-Fucosyllactose is authorized for placing on the market within the EU only by Glycom A/S, unless:
- a subsequent applicant obtains authorization for the novel food without reference to the proprietary scientific evidence or scientific data protected in accordance with Article 26 of Regulation (EU) 2015/2283 or;
- with the agreement of “Glycom A/S”.
Authorized usage scopes and maximal usage levels:
Authorized novel food | Conditions under which the novel food may be used | |
3-Fucosyllactose (‘3-FL’) (produced by derivative strain of E. coli K-12 DH1) | Specified food category | Maximum levels |
Infant formula as defined under Regulation (EU) No 609/2013 | 1.75 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer | |
Follow-on formula as defined under Regulation (EU) No 609/2013 | 1.75 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer | |
Unflavoured pasteurised and unflavoured sterilised (including UHT) milk products | 2.0 g/L | |
Unflavoured fermented milk-based products | 2.0 g/L (beverages) | |
4.0 g/kg (products other than beverages) | ||
Flavoured fermented milk-based products including heat-treated products | 2.0 g/L (beverages) | |
12.0 g/kg (products other than beverages) | ||
Cereal bars | 25.0 g/kg | |
Milk based drinks and similar products | 2.0 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer | |
12.0 g/kg (products other than beverages) | ||
Beverages (flavoured drinks, excluding drinks with a pH less than 5) | 1.25 g/L | |
Total diet replacement foods for weight control as defined under Regulation (EU) No 609/2013 | 2.0 g/L (beverages) | |
25.0 g/kg (products other than beverages) | ||
Foods for special medical purposes as defined under Regulation (EU) No 609/2013 excluding foods for infants and young children | In accordance with the particular nutritional requirements of the persons for whom the products are intended but in any case not higher 4.0 g/L or 4.0 g/kg in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer. | |
Food Supplements as defined in Directive 2002/46/EC, for the general population, excluding infants and young children | 4.0 g/day | |
Additional specific labeling requirements | ||
| ||
6’-Sialyllactose sodium salt produced by derivative )strain of Escherichia coli W (ATCC 9637)

On October 24, 2023, the European Commission issued the COMMISSION IMPLEMENTING REGULATION (EU) 2023/2215, and authorizing the placing on the market of 6’-Sialyllactose sodium salt produced by derivative ) strain of Escherichia coli W (ATCC 9637) as a novel food and amending Implementing Regulation (EU) 2017/2470. This Regulation entered into force on November 13, 2023.
Applicant: Kyowa Hakko Bio Co., Ltd
By the end of November 13, 2028 (data protection period), the novel food 6’-Sialyllactose sodium salt is authorized for placing on the market within the EU only by Kyowa Hakko Bio Co., Ltd, unless:
- a subsequent applicant obtains authorization for the novel food without reference to the proprietary scientific evidence or scientific data protected in accordance with Article 26 of Regulation (EU) 2015/2283 or;
- with the agreement of “Kyowa Hakko Bio Co., Ltd”.
Authorized usage scopes and maximal usage levels:
Authorized novel food | Conditions under which the novel food may be used | |
‘6’-Sialyllactose (6’-SL) sodium salt (produced by derivative strain of E. coli W (ATCC 9637)) | Specified food category | Maximum levels |
Unflavoured pasteurised and unflavoured sterilised (including UHT) milk products | 0.5 g/L | |
Unflavoured fermented milk-based products | 0.5 g/L (beverages) | |
2.5 g/kg (products other than beverages) | ||
Flavoured fermented milk-based products including heat-treated products | 0.5 g/L (beverages) | |
5.0 g/kg (products other than beverages) | ||
Beverages (flavoured drinks, excluding drinks with a pH less than 5) | 0.5 g/L | |
Cereal bars | 5.0 g/kg | |
Infant formula as defined under Regulation (EU) No 609/2013 | 0.4 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer | |
Follow-on formula as defined under Regulation (EU) No 609/2013 | 0.3 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer | |
Processed cereal-based food and baby food for infants and young children as defined under Regulation (EU) No 609/2013 | 0.3 g/L (beverages) in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer | |
2.5 g/kg for products other than beverages | ||
Milk based drinks and similar products | 0.3 g/L (beverages) in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer | |
Total diet replacement foods for weight control as defined under Regulation (EU) No 609/2013 | 1.0 g/L (beverages) | |
10.0 g/kg (products other than beverages) | ||
Food for special medical purposes as defined under Regulation (EU) No 609/2013 | In accordance with the particular nutritional requirements of the persons for whom the products are intended | |
Food Supplements as defined in Directive 2002/46/EC, excluding food supplements for infants and young children | 1.0 g/day | |
Additional specific labeling requirements | ||
| ||
Note: The data in this article is for reference only. Please refer to the official information published by Official Journal of the European Union.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information
Latest Compliance Advances of Human Milk Oligosaccharides (HMOs) in China

