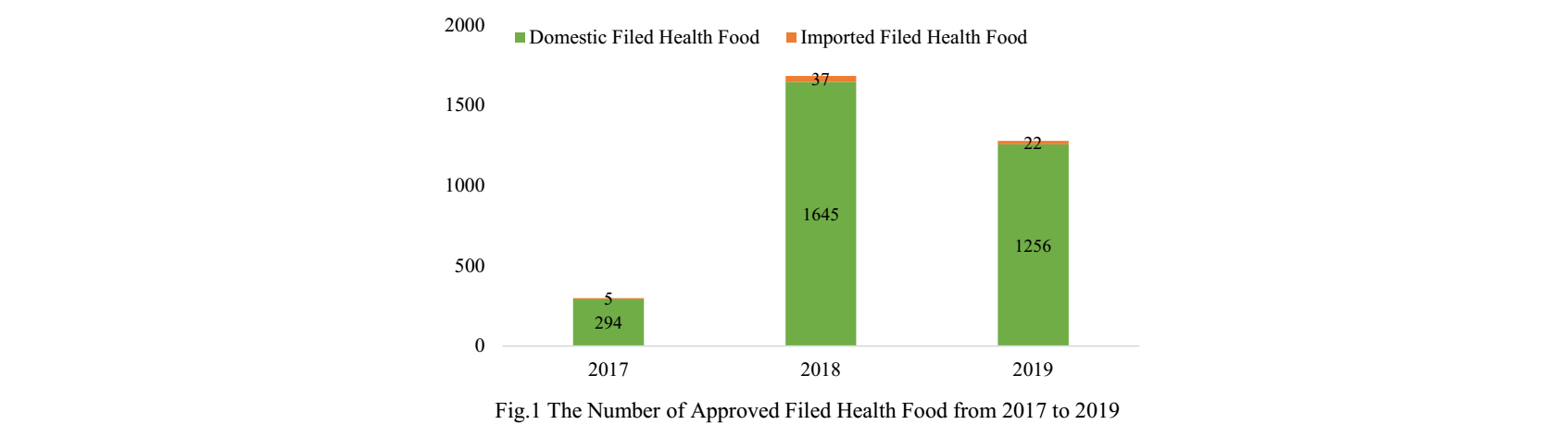
The Administrative Measures for the Registration and Filing of Health Food (hereinafter named “the Measures”) was implemented on July 1st, 2016. Since the first filed health food was approved in 2017, more than three thousands health foods have obtained filing certificates by the end of 2019. In order to help relevant enterprises have an overview of this kind of products in China, CIRS counted the data of the approved domestic and imported health food filing products from 2017 to 2019, and made an analysis for your reference.
1. The Number of Approved Filed Health Food in China
Since former Guangdong FDA issued the first domestic filed health food on 27th July, 2017, the number of approved filed health foods has reached 3259 by Dec. 31st, 2019. The quantity of domestic and imported filed health foods is 3195 and 64 respectively.
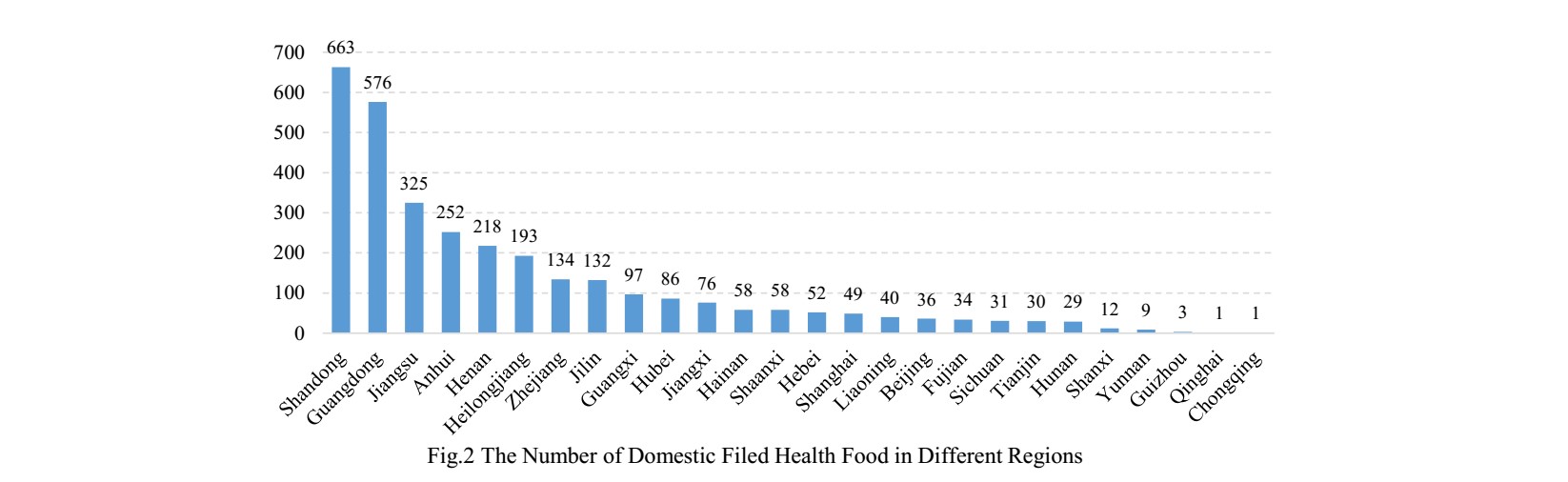
2. The Number of Approved Filed Health Food in Different Regions
2.1 Domestic Nutrition Supplement
The number of approved domestic filed health foods is varied in different provinces in China. Shandong and Guangdong rank the first and second place respectively with the number of 663 and 576, which account for 20.75% and 18.03% of total respectively.
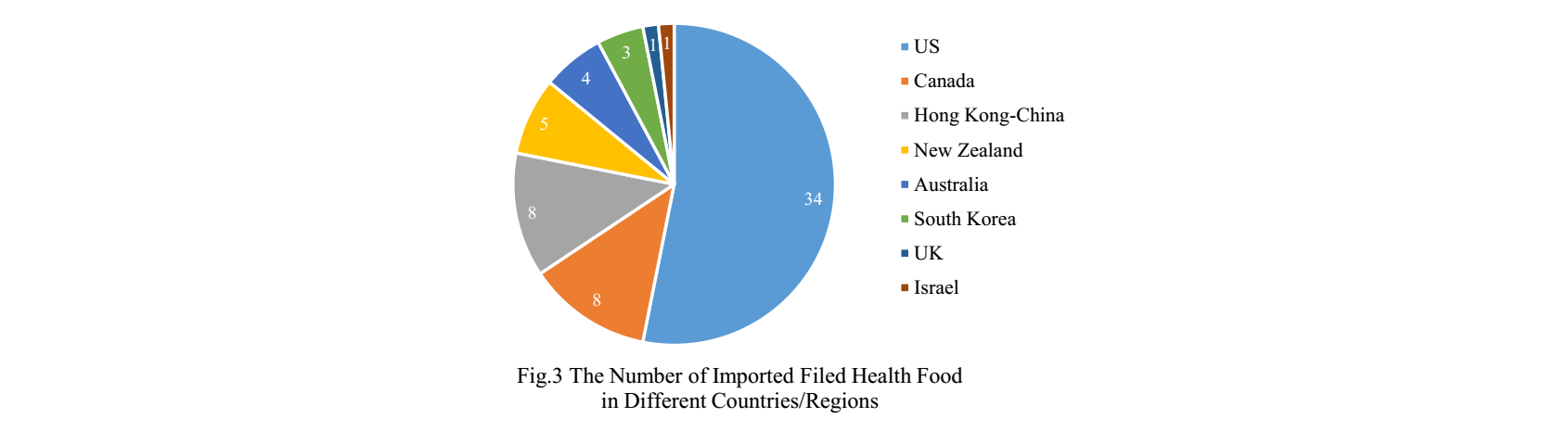
2.2 Imported Nutrition Supplement
The applicants who come from the United States, Canada, Hong Kong-China, New Zealand, Australia, South Korea, the United Kingdom and Israel have obtained filing certificates, and 34 of total approved imported nutrition supplements are from American companies. It is the first time for the UK and Israeli companies to get filing certificates in 2019.
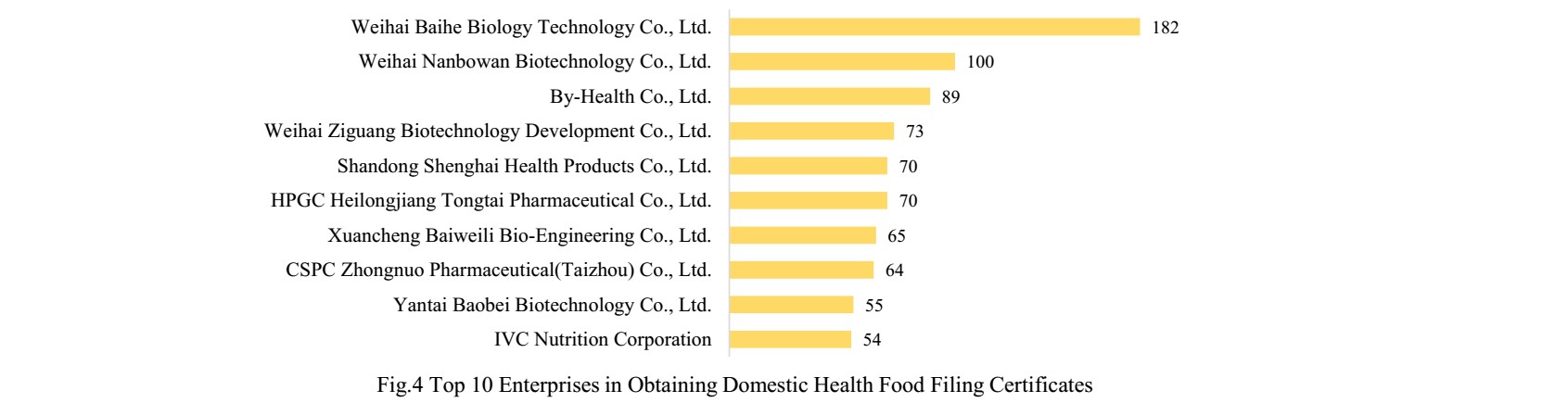
3. Enterprises in Obtaining Health Food Filing Certificates
3.1 Domestic Nutrition Supplement
There are 363 domestic health food manufacturers who have obtained health food filing certificates. Among them, Weihai Baihe Biology Technology Co., Ltd. has got the largest number of filing certificates (182), followed by Weihai Nanbowan Biotechnology Co., Ltd. and By-Health Co., Ltd. with the number of 100 and 89, respectively.
3.2 Imported Nutrition Supplement
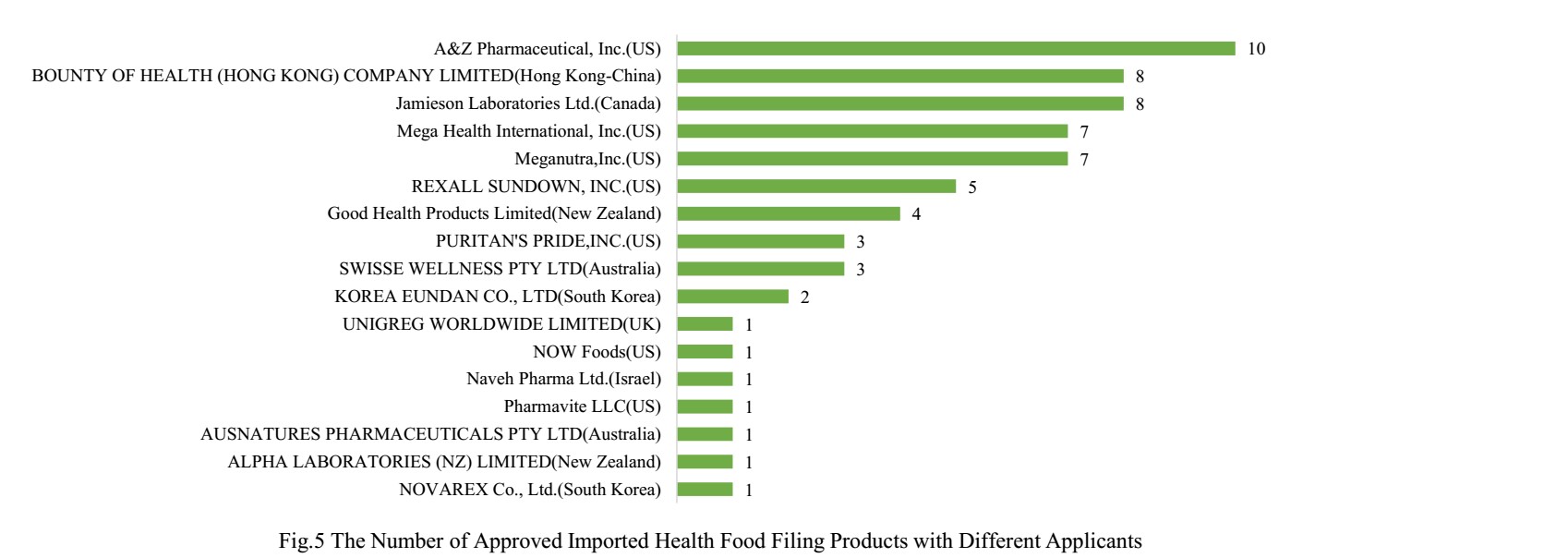
There are 17 oversea companies who have got health food filing certificates, A&Z Pharmaceutical, Inc. has got the largest number of filing certificates (10). BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED and Jamieson Laboratories Ltd. rank the second place with the number of 8 products, followed by Mega Health International, Inc. and Meganutra, Inc., which is 7 products respectively.
4. The Number of Approved Filed Health Food with Different Dosage Forms
There are 5 dosage forms for nutrition supplement which can apply for filing certificate in China currently, namely, tablet, hard capsule, soft capsule, oral liquid (including drops) and granule.
4.1 Domestic Nutrition Supplement
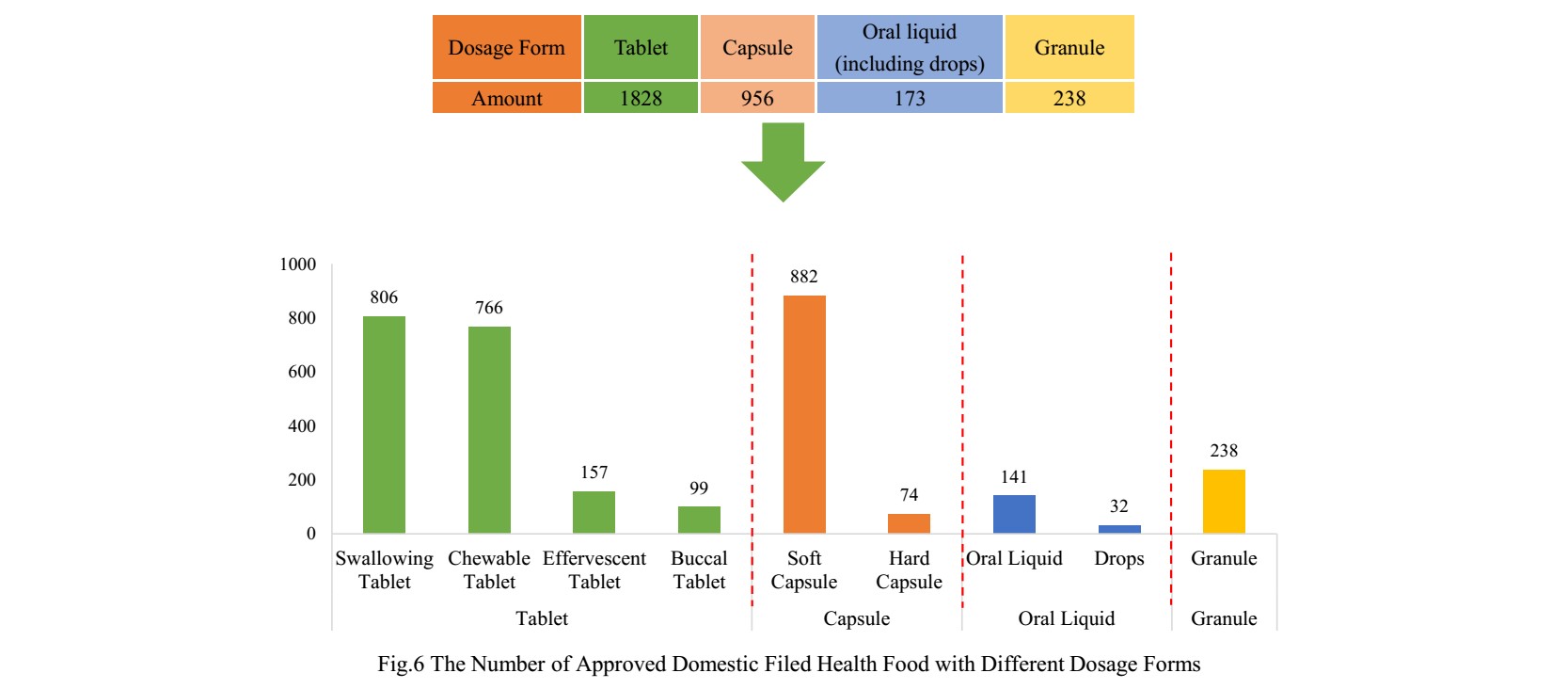
The dosage forms of approved domestic nutrition supplements are mainly tablets with the number of 1828, which account for 57.25% of total. Among them, the number of products with the dosage of swallowing tablets and chewable tablets is the largest, which has reached 806 and 766. However, the quantity of products with effervescent tablets and buccal tablets is only 157 and 99.
Capsule include soft capsule and hard capsule, the number of products with capsules is 956. The quantity of soft capsule products is far higher than the number of hard capsule products, which is 882 and 74 products respectively.
In addition, the quantity of oral liquid (including drops) and granule products is 173 and 238. And there is only 32 dorps products.
4.2 Imported Nutrition Supplement
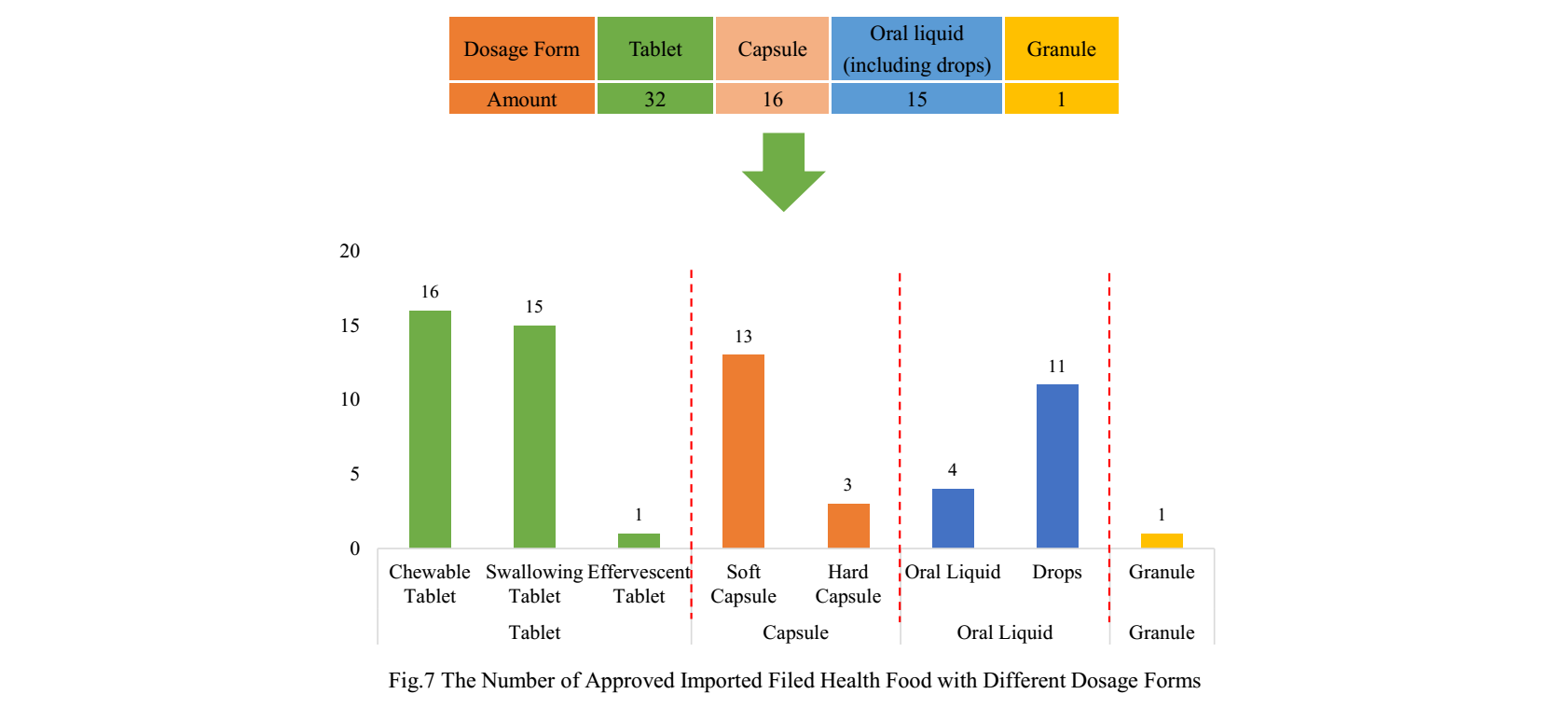
The dosage forms of approved imported nutrition supplements are mostly tablets with the number of 32, which account for 50% of total. Among them, the number of chewable tablet products and swallowing tablet products is the largest, and there is only 1 product with the dosage of effervescent tablets. Besides, there is no buccal tablets product currently.
The quantity of capsule products and oral liquid (including drops) products is 16 and 15 respectively. The same as domestic nutrition supplements, the quantity of soft capsule products is far higher than the number of hard capsule products. Different from domestic nutrition supplements, the number of imported drop products is more than oral liquid products. There is only 1 granule product for now.
5. The Number of Approved Filed Health Food with Different Nutrients
5.1 Domestic Nutrition Supplement
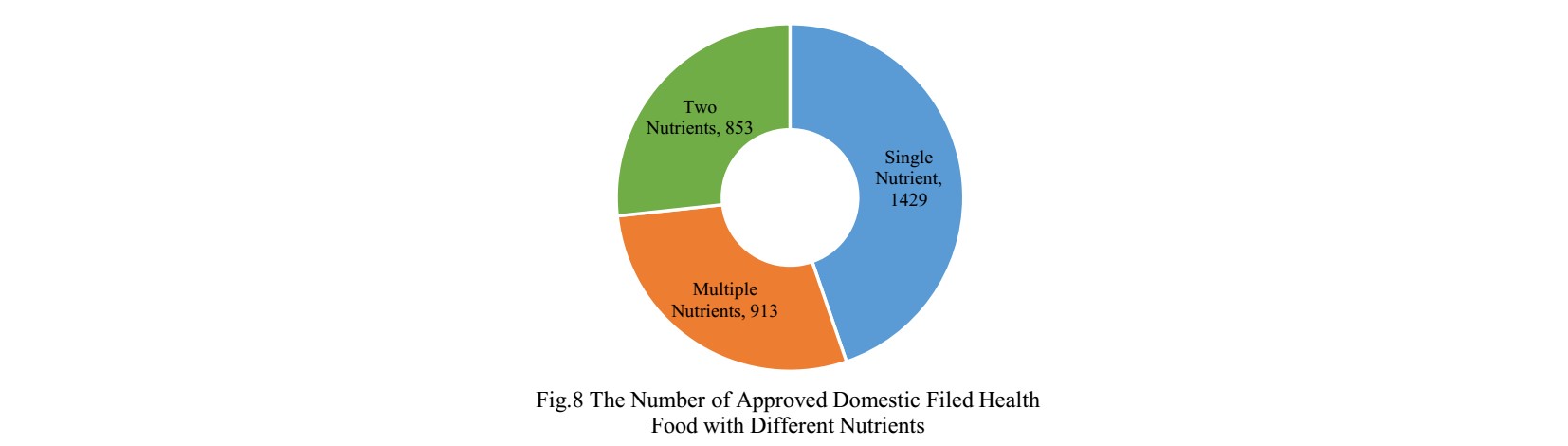
The number of domestic products supplementing single nutrient is the most, which is 1429, followed by the products supplementing multiple nutrients and two nutrients.
Among domestic health food filing products, the
most popular nutrition supplements are multi-vitamins and minerals supplements,
Vitamin C supplements, Calcium and Vitamin D supplements, which is 531, 486 and
314 respectively.
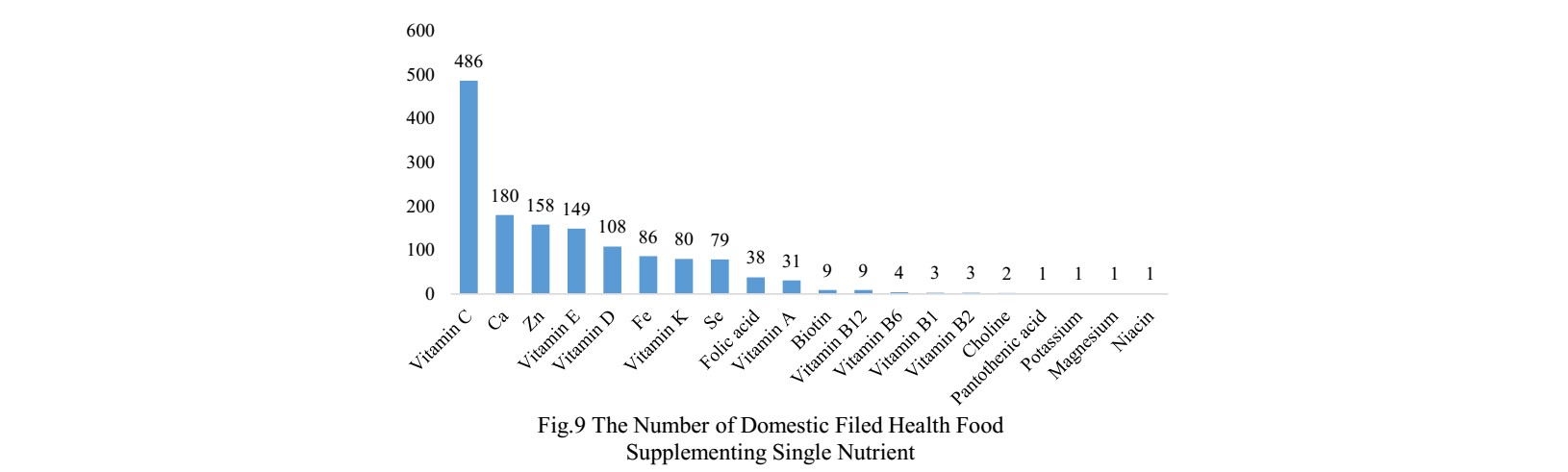
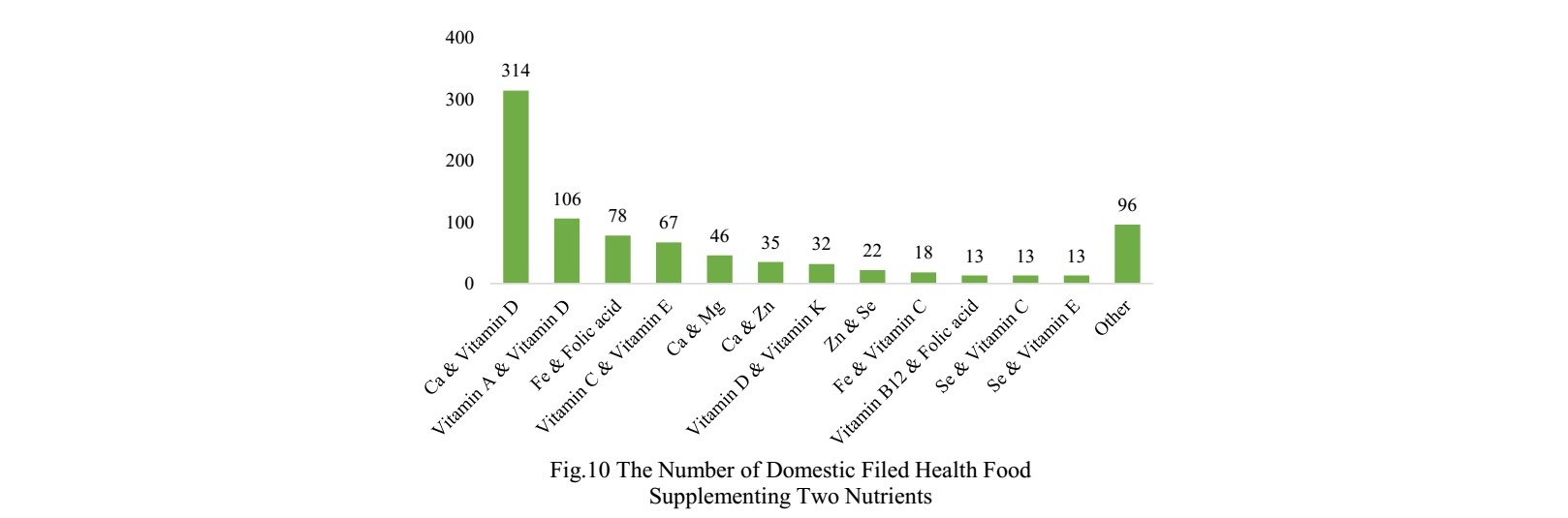
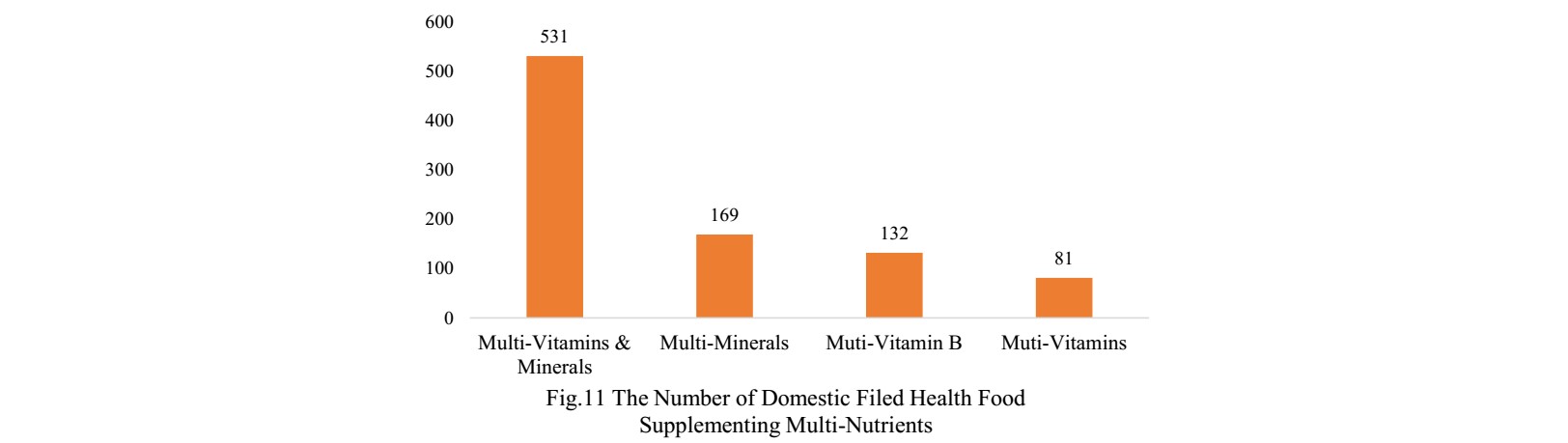
PS. Nutrients with less than 13 products are classified as “Other”.
5.2 Imported Nutrition Supplement
Among imported health food filing products, the most popular nutrition supplements are multi-vitamins and minerals supplements, Calcium and Vitamin D supplements, and Vitamin D supplements, which is 9, 9 and 7 respectively.
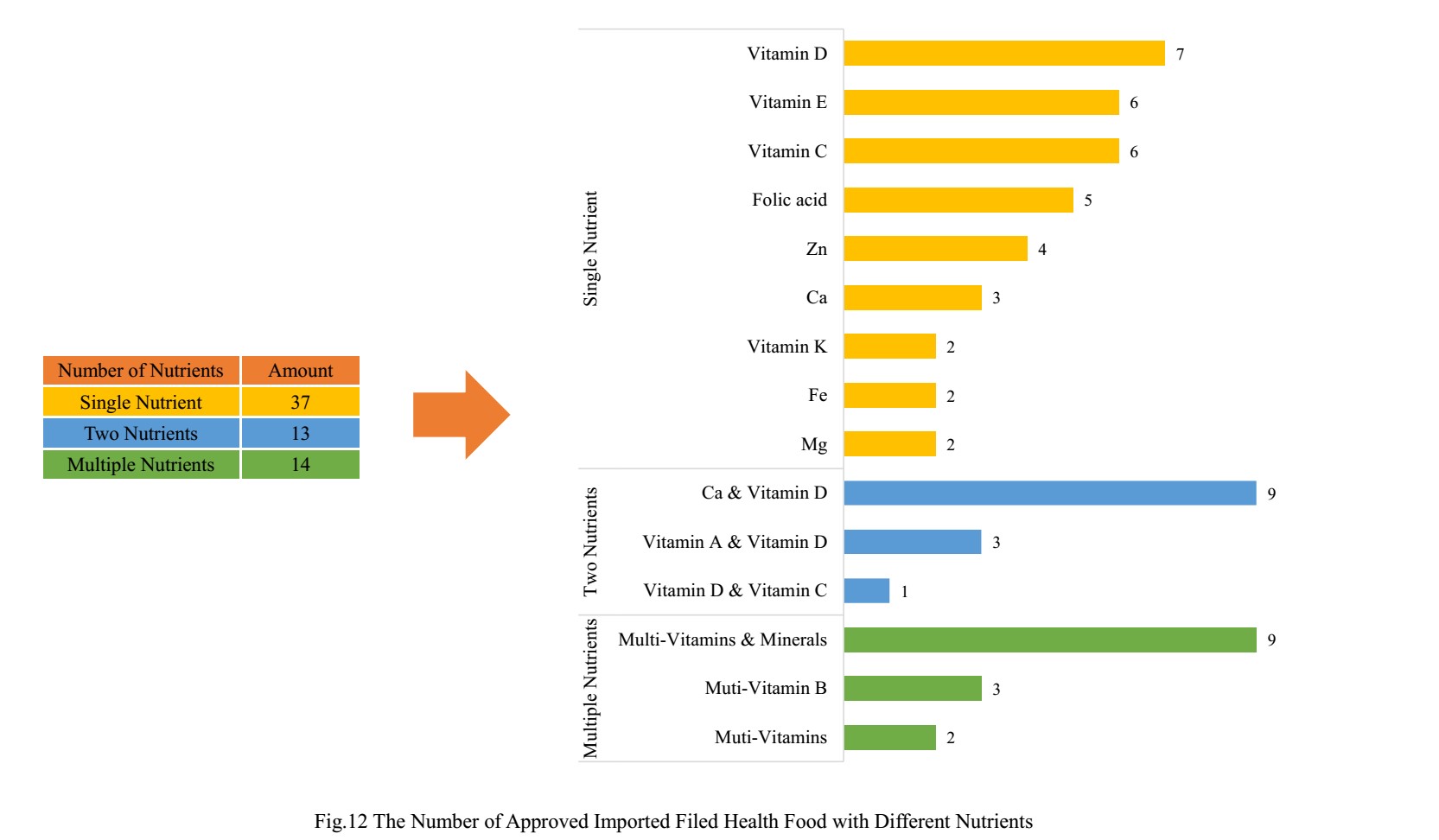
6. CIRS Comments
The implementation of the health food filing has simplified the procedures of administrative approval and reduced the reporting period and costs, so it is favored by health food companies. From 2017 to 2019, more than three thousand nutrition supplements have obtained filing certificates. While only 64 imported products have got filing certificates, far fewer than domestic products. As for the main reasons why the imported dietary supplements cannot be filed smoothly, CIRS believes that due to the lack of fully understanding of Chinese laws and regulations related to health food, the relevant supporting documents sometimes cannot meet the requirements of the Chinese laws and regulations, resulting in a relatively low pass rate.
While health food enterprises are glad to obtain filing certificates, the pressure of homogeneous product competition comes one after another. From another point of view, the filing system may lead manufacturers to producing similar products due to the restrictive raw materials and excipients, and the competition will be getting tougher. Therefore, how to highlight the advantages of products is where enterprises need to consider.
The release of Available Excipients for Heath Food Filing and Their Usage Rules (2019) has brought new hopes to health food companies. Compared with the Available Excipients for Heath Food Filing and Their Usage Rules (2017) , the new excipients directory added 17 available excipients, including common food additives such as phospholipids, Octyl and decyl glycerate, and common food materials such as fruit and vegetable powder. These new excipients provide a more favorable guarantee for enriching the taste of the products and enhancing the market competitiveness of the products. Besides, the new standards of 28 excipients also provide more space for companies to choose suppliers. In addition, afterCoenzyme Q10, Melatonin, Fish oil, Broken Ganoderma lucidum spore powder and Spirulina Health Food Raw Materials Directory and Their Technical Requirements (Draft for public comments) is officially passed, enterprises will have more space to choose raw materials and function claims for filing products.
PS. There may be some omissions in the data of domestic filed health food due to the replacement of new and old websites of government departments after the reform of state institutions. The data in this paper is for reference only. Please refer to the information published by the government.

