In recent years, Human milk oligosaccharides (HMOs) has become the new aristocrat in milk and been well known by the public. With the development of science and technology and the deepening of research, people are increasingly aware of its importance. HMOs are the third largest solid component in breast milk following lactose and fat; and a unique group of complex mixed carbohydrates found naturally in breast milk. Numerous studies have shown that HMOs can promote the growth of beneficial bacteria and provide unique nutritional value for human body. Therefore, domestic and foreign enterprises have started the research and development of infant formula milk powder matching with “breast milk nutrition”.
The importance of HMOs in the field of food nutrition has been constantly confirmed. Its typical ingredient 2'-FL has obtained the "pass" in the European Union, the United States, Australia and New Zealand successively, and available for commercial us. Although 2'-FL has not been officially approved for use in China, many 2'-FL applications have been reviewed for public comments by the China National Center for Food Safety Risk Assessment (CFSA), including synthetic sources and modified E.coli sources, which indicates that the approval of 2'-FL in China is just around the corner. Being a new functional ingredient, what is the current regulatory status for 2'-FL; what is the prospect of industrial research and application of 2'-FL? CIRS Group takes you to find out in the following.
1. 2'-FL introduction
2'-FL is a neutral fucosylated oligosaccharide in human milk, accounting for about 31% (mole fraction) in HMOs, with a content of 0.06-3.93 g/L, which is the highest relative abundance oligosaccharide in human milk [1]. The monomers consist of fucose, galactose and glucose.
English name: 2' -fucosyllactose, 2' -o-fucosyllactose (2'-FL)
Chemical name: α -L-pyran fucosyl -(1 2)-β -D-pyran galactosyl -(1 4) -D-glucopyranoside
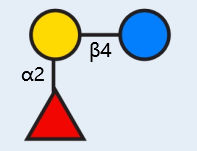
Structure of 2'-FL
 Fucose,
Fucose,  Galactose,
Galactose,  Glucose
Glucose
Photograph: Nutrition Reviews
2. Current regulatory status of 2'-FL
2' -FL is the highest oligosaccharide in human milk and one of the first HMOs approved by FDA and EU to be used in infant milk powder, common food, dietary supplements and/or medical food. Meanwhile, 2'-FL produced by microbial fermentation has been approved for use in Australia and New Zealand as well.
- USA
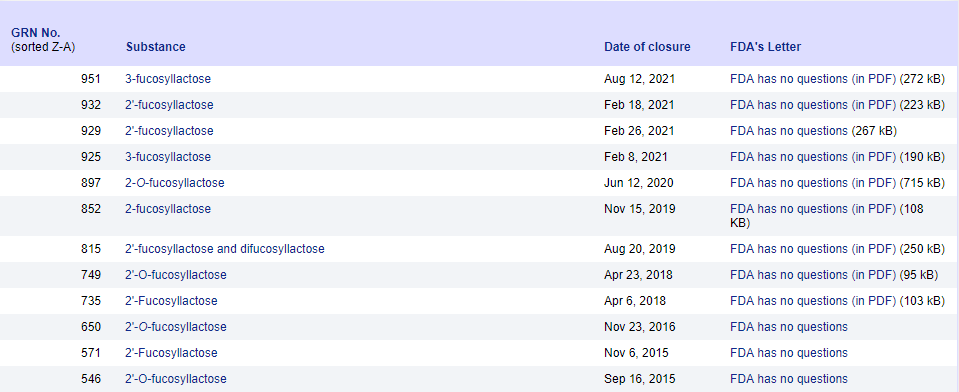
To date, several 2'-FL (single ingredient or combination products) have been approved by FDA as "Generally recognized as safe" (GRAS) substances. The scope of use includes infant formula milk powder and baked goods, dairy products, beverages and other general food categories; the amount of use varies according to different units.
Photograph: US FDA
- EU
In accordance with Regulation of the European Commission (EU) 2017/2470, 2' -FL has been approved as a new Food ingredient (Novel Food). The production source includes synthetic, transgenic E. coli BL21 strain and transgenic E. coli K-12 strain. The range of use includes infant formula, dietary supplements, fermented dairy products, coffee, tea, etc., with a maximum of 1.2 g/L alone in infant and larger infant formula (calculated by ready-to-use products).
- Australia and New Zealand
The Food Standards Australia New Zealand (FSANZ) first approved the use of 2'-FL and LNnT, produced by microbial fermentation, for infant formula products and formulated supplementary foods for young children in December 2019. On 8 November 2021, FSANZ issued an approval report on the application of 2'-FL produced by fermentation of a genetically modified strain of E. coli for use in infant formula, larger infant formula and formulated supplementary foods for young children.
3. 2'-FL industrialization research status
2'-FL is a typical representative of neutral fucosylated HMOs, with the highest relative abundance in human milk, and has a variety of functional activities such as regulating intestinal flora, resistance to pathogen adhesion, immune regulation, and promotion of nervous system development and repair [2]. At present, commercial 2'-FL production mainly includes chemical synthesis, enzyme-catalyzed synthesis and microbial fermentation production [3].
Chemical synthesis requires high cost of raw materials, and the production process is more complicated and time-consuming. Enzymatic synthesis needs specific fucose transferase. Enzymatic synthesis of 2'-FL has mild and controllable reaction conditions, short time and easy purification of the product, but the cost of glycosyltransferase donor is high, which largely depends on the discovery of protein engineering to improve the efficiency of glycosyltransferase. Industrial production is still some way off. [4].
Microbial fermentation is the main method of industrial production of 2'-FL at present, which is biosynthesis of 2'-FL based on its own (or simulated) metabolic mechanism. In this process, genetic engineering techniques are used to express enzyme fragments that were originally lacking in the original host cell to add missing functions. This method of production is relatively low cost and can be mass produced.
4. 2'-FL is poised for approval in China
As mentioned before, the vast majority of 2'-FL products that have received FDA GRAS approval are from genetically modified microbial sources, including genetically modified E.coli and corynebacterium glutamicus. The European Union has also approved 2'-FL sourced from genetically modified microorganisms. In China, in fact, before 2020, the safety assessment procedures of new food raw materials or new food additives involving genetically modified technology were not clear, except that enzyme preparations derived from genetically modified microorganisms can be applied as new food additives in China.
As early as August 10, 2016, some enterprises tried to register 2’FL as a new food additive (nutrition enhancer) used in infant formula food. On August 15 of the same year, the CFSA launched a public review and solicitation of opinions on the contents that could be disclosed to the public (without CFSA technical review). But since then, news on the review of this kind of product has been largely ignored.
In 2021, the National Health Commission opened the application path for GMO-derived food additives in China. For detailed application procedures and material requirements for new GMO-derived food additives, please refer to Genetically Modified Microorganism Food Additive Registration in China.
This policy paves the way for compliant use in China of HMOs produced by genetically modified microbial fermentation. In the second half of 2021, three companies submitted applications for new GMO-derived 2 '-FL. In recent days (April 15, 2022), two strains of 2'-FL from modified E.coli have been reviewed by experts and are released for public comments. See the article for details. Although 2'-FL produced from chemical synthesis was released for public comments in October 2021, it was postponed again and is still in the data correction stage.
It is believed that 2' FL will soon get a "passport" in China. By then, the Infant nutrition market in China will continue to be explored, and the gap between infant formula and breast milk will gradually be filled.
If you have any needs or questions, please contact us at service@cirs-group.com
Our service
- NHC New Food Raw Material Registration in China
- NHC New Food Additive Registration in China
- Genetically Modified Microorganism Food Additive Registration in China
Reference
[1] BYCH K, MIKS M H, MARKUS T J, et al. Production of HMOs using microbial hosts — from cell engineering to large scale production [J]. Current Opinion in Biotechnology, 2019, 56C: 130-137.
[2] VANDENPLAS Y, BERGER B, CARNIELLI V P, et al. Human milk oligosaccharides: 2'-fucosyllactose (2'-FL) and lacto-N-neote-traose (LNnT) in infant formula [J]. Nutrients, 2018, 10(9): 1161.
[3] RAN SHI, ZHENGQIANG JIANG. Enzymatic synthesis of 2'-fucosyllactose: advances and perspectives [J]. Synthetic Biology Journal 2020, 1(4): 481-494.
[4] BODE L, CONTRACTOR N, BARILE D, et al. Overcoming the limited availability of human milk oligosaccharides: challenges and opportunities for research and application [J]. Nutrition Reviews, 2016, 74(10): 635-644.

