According to the "Regulations on the Supervision and Administration of Medical Devices" (No. 739), medical devices are classified and managed according to the degree of risk.
Class I medical device is a medical device with a low degree of risk, and the implementation of routine management can ensure its safety and effectiveness. Overseas medical device manufacturers need to entrust a domestic agent to file with the NMPA.
Regulation:
"Regulations on the Supervision and Administration of Medical Devices" (No. 739)
Service Process:
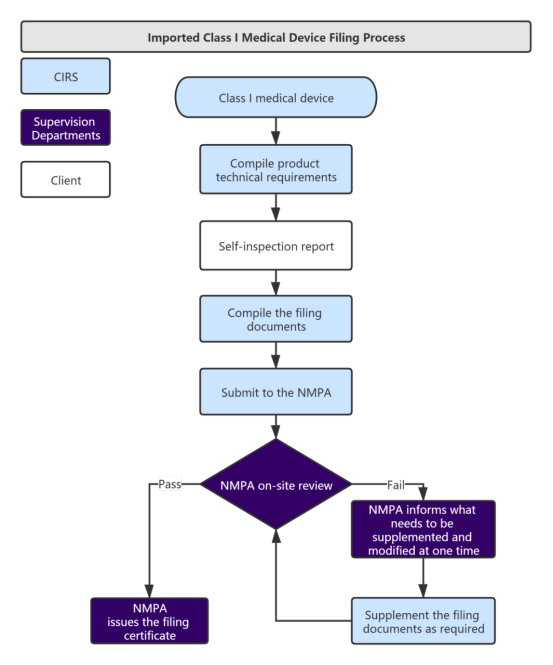
Administrative Fee:
Free
Time distribution:
Accept and review on the spot, if the document needs to be supplemented and revised, applicants will be notified on the spot.
Related service:
Product classification determination
Class I medical device filing and filing change
Class II/III medical device registration, registration change and renewal
Product testing and rectification technical support
Technical files compilation
Medical device registration under the MAH system
Registration of imported-to-domestic products
Follow-up and correction of medical device registration technical review