The guideline applies for application and handle of registration renewal of imported medical device.
2. Item Information
Project Name: Examination and approval for imported medical device registration;
Sub-project Name: Examination and approval for registration renewal for imported medical device.
3. Regulation Basis
The article 15 of the Supervision and Administration of Medical Device has stated that the valid period of medical device registration is 5 years. If manufacturer want to renew their certificate, shall apply for certificate renewal to CFDA at least 6 months before certificate expiration.
4. Accepting institution
Center for Medical Device Evaluation. CFDA (hereafter refers as MDE)
5. Decisive institution
China Food and Drug Administration
6. Application condition
The applicant shall be oversea manufacturer, and the medical device has been registered by CFDA.
7. Application Documents
| Document List | Description |
| 1.Application | |
| 2.Supporting Documents | Oversea manufacturer shall submit the documents such as Power of attorney of agent, letter of commitment of agent, and business license. |
| 3. A statement about no change of medical device. | |
| 4.The copy of original registration certificates and its attachments of medical devices; | |
| 5. Medical device analysis report in valid period | 5.1 Clinical application status, consumer complaint and response action. 5.2 Evaluation report of adverse event. 5.3 A statement of market condition 5.4 Supervision and selective testing status (if any). 5.5 If the product has been recalled, please explain the reason, process and result. 5.6 The work specified in the original certificate that to continue in the future shall be complete which you renew your certificate. |
| 6. Product inspection report | 1.If the mandatory standard of medical device has been revised, you shall submit the new test report which can prove your product meet the requirements of new mandatory standards. |
| 7. Declaration of conformity | |
| 8. Other |
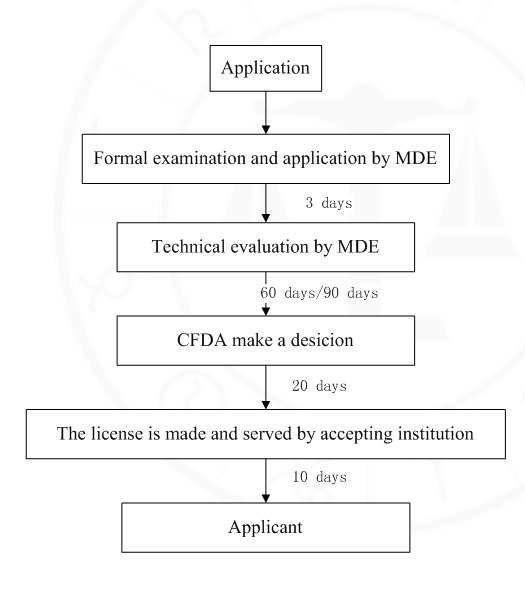
8. Handling Process and time limit
9. Fee Standard
Medical device of Class II and Class III: RMB 40,800.
10 Approval result
10.1 For medical device

10.2 For IVDS


