Leachable Substances are chemical substances released from medical devices during the continuous contact and function of medical device products with the human body, or when interacting with other media in use (such as blood, liquid medicine, etc.). While medical devices are playing a role, the leachables also cause safety hazards to the human body in the short or long term. Therefore, its safety assessment is not only the content that enterprises need to pay attention to in the design and development phase of products, but also the focus of technical review of related products.
Safety Evaluation Services of E&L Assessments:
- Material selection/process optimization evaluation
- Toxicological risk assessment
- Equivalence evaluation (of proposed material to a clinically established material)
- New material screening and selecting
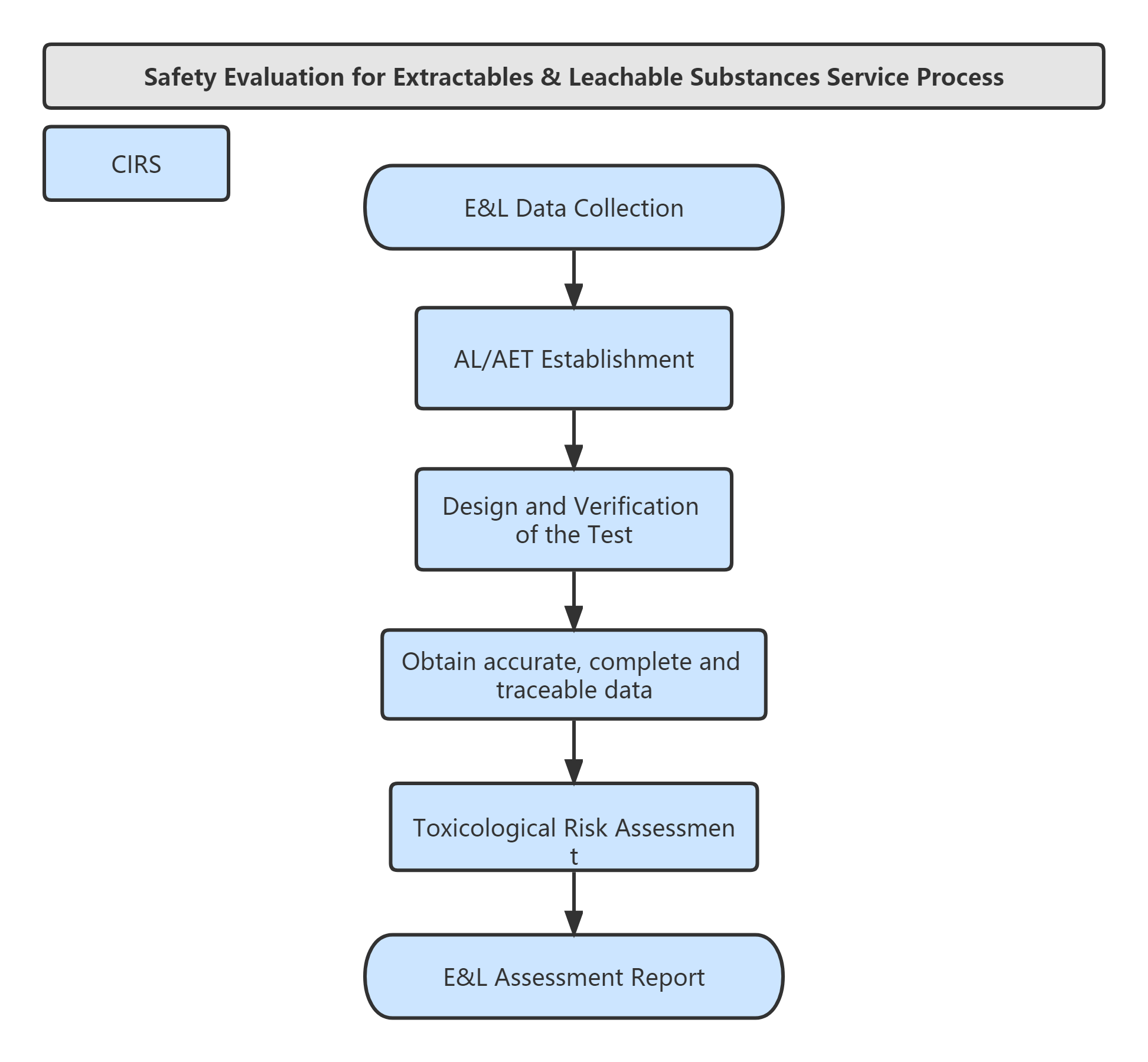
Service Process: