In the year of 2016, CFDA has processed 8920, reviewed 9336 and approved 8653applications in total. For all medical devices and in vitro diagnostic reagents(IVD), companies who plan to place medical products on Chinese market must apply for and acquire Medical Device Registration Certificate (Chinese:医疗器械注册证) from CFDA. Foreign firms should designate local legal agent and service agent to deal with registration and after-sales service if there is not subsidiary or representation office in China, the more information on how to register your products in China can be found here.
Note, the Annual Report for Medical Device Registration in 2015 can be found here.
1.Overall situation
In the year of 2016,CFDA had processed total of 8920 applications of medical device registration, continued registration and modification of permitted affairs. It is reduce about 5.1% compared with 2015.Reviewedapplications was a total of 9336 applications, increased 0.25% compared with 2015.Approved applications was 8653,increased 14.9%, compared with 2015.
2.Itemization situation
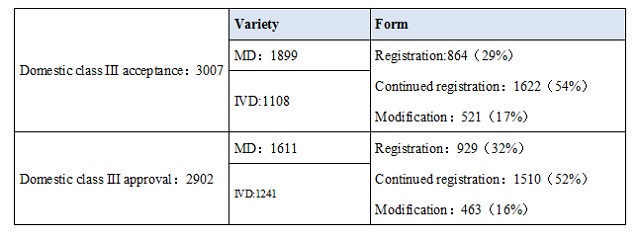
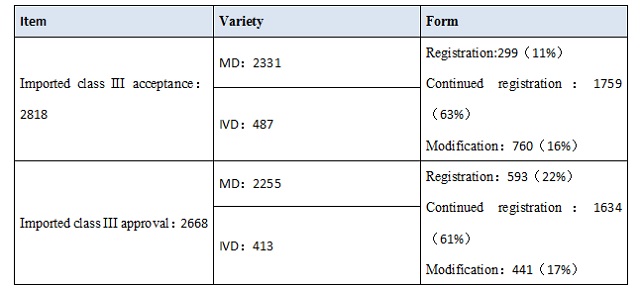
The detailed condition of domestic class III、Imported class II、Imported class III was listed.

3.Species analysis of approved medical device
From the date, it’s obvious that IVD accounted for a large proportion of registered products. In domestic class III medical device, IVD accounted for 43%,and in imported, it takes 33%.
Expect for IVD, domestic medical device of class III registration concerned 28 sub-directories of “Classification of medical devices” in the year of 2016.
The registration number of the top five domestic medical device of class III are: implants and artificial organs, medical polymer materials and products, injection puncture apparatus, medical optical apparatus ,instruments and endoscopic equipment, equipment and apparatus in operation room, emergency room treatment, laboratory.
Compared with 2015, implant materials and artificial organ products rose from second to the first place, while the equipment and appliances in operation room, emergency room, and laboratory replaced the intervention equipment.
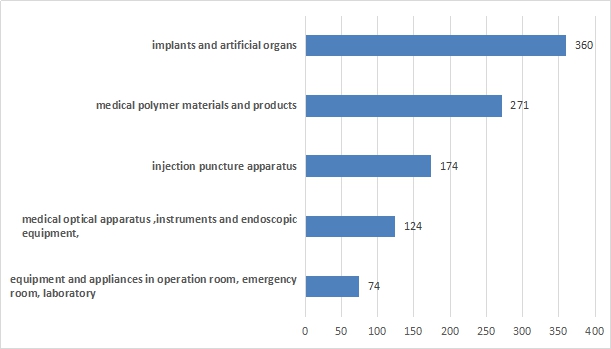
Figure 1Specie ranking of domestic medical device of classIII
Expect for IVD, imported medical device registration concerned 39 sub-directories of “Classification of medical devices”, in the year of 2016.
The registration number of the top five imported medical device are: implants and artificial organs, medical polymer materials and products, medical electronic instrument and equipment, medical optical apparatus ,instruments and endoscopic equipment, equipment and apparatus in operation room, emergency room treatment, laboratory.
Compared with 2015, implant materials and artificial organ products rose from second to the first place, while the equipment and appliances in operation room, emergency room, and laboratory replaced the department of Stomatology materials.
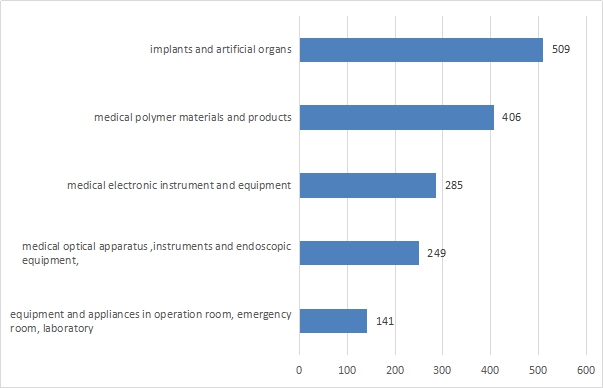
Figure 2 Specie ranking of imported medical device
4. Source analysis of imported medical devices
The same as 2015, the countries of top five imported medical device are America, German, Japan, English, and Korea, make up 67% of first and renew registration in 2016.
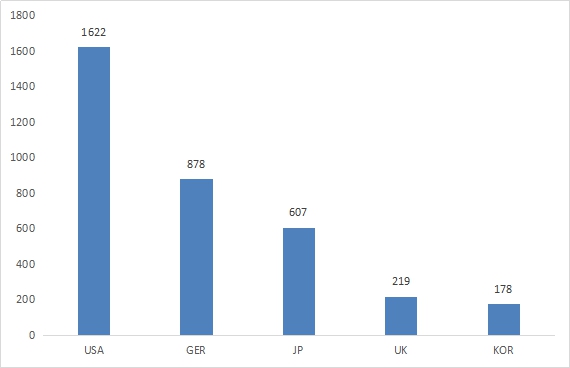
Figure 3 Country Ranking of Imported Medical Device
If you have any other needs or questions, please contact us at MD@cirs-group.com or join the webinar about The Latest Update of Medical Device Regulations in China on 26th April 2017.

