
The health risk assessment report of pesticides is not only an important part of pesticide registration materials but also an important basis for China's Institute Control of Agrochemicals, Ministry of Agricultural and Rural Affairs (ICAMA) to decide whether to approve pesticide registration in China. Scientifically speaking, pesticide health risk assessment is based on scientific data and models – scientifically evaluating the possibility and severity of adverse effects of pesticides on human health under specific conditions. This article summarizes the key technical points and precautions in conducting pesticide health risk assessment in China.
Basic Process of Pesticide Health Risk Assessment
Pesticide health risk assessment generally includes hazard identification, hazard assessment, exposure assessment, and risk characterization, which are also the four main steps of the pesticide health risk assessment report.
At the hazard identification stage, we need to clarify the protection target of risk assessment, identify the main exposure routes, explain the risk issues in detail, and determine the purposes of risk assessment.
At the hazard assessment stage, we need to collect and evaluate the toxicological data of the technical AIs and finished formulations, consider the interspecies differences between experimental animals and humans and the individual differences within the population, and use appropriate uncertainty factors (UF) to derive the allowable operator exposure levels (AOEL or AREL) for pesticide operators or residents.
At the exposure assessment stage, considering the influence of factors such as formulation type, application method, and environmental conditions, the unit exposure method or assessment model is used to calculate the predicted exposure levels (Exposure) of operators or residents.
At the risk characterization stage, we use the predicted exposure level (Exposure) divided by the AOEL or AREL to obtain the risk coefficient (RQ). If RQ<1, the health risk is generally considered acceptable.
Hazard Identification
The most important task of the hazard identification stage is to identify whether the pesticide has the possibility of posing a health hazard to relevant personnel, determine the protected population, and health risk assessment projects. According to the different protection populations, pesticide health risk assessment is currently mainly divided into operator health risk assessment and residential health risk assessment.
For operators, we generally consider dermal and inhalation exposure risks; for residential health risk assessment, we also need to consider oral exposure risks. Dietary risk assessment for consumers also belongs to health risk assessment, but it is not within the scope of this article because it has a separate technical guide. Generally speaking, field-use pesticides and environmental health pesticides require operator health risk assessment, while household health pesticides require residential health risk assessment. It is worth noting that the main difference between environmental health pesticides and household health pesticides is not whether the product is used indoors or outdoors, but whether the product needs to be diluted before use.
Hazard Assessment
The two most important tasks in the hazard assessment stage are the comprehensive evaluation of toxicological data and the calculation of AOEL or AREL. Although we generally use subacute or sub-chronic toxicity data to derive allowable exposure levels, this does not mean that we only need to collect subacute and sub-chronic toxicity data and evaluate them.
NY/T 3153-2017 "Guidelines for Health Risk Assessment of Pesticide Operators" requires a comprehensive analysis and evaluation of the toxicological data of pesticide active ingredients and formulations, and special attention should be paid to whether pesticides have special toxic effects such as mutagenicity, reproductive and developmental toxicity, carcinogenicity, and neurotoxicity. In simple terms, whether using in-house test data or referring to external literature data (such as EFSA, and EPA data), the toxicological information summary in section 3.1 of the pesticide health risk assessment report should be as comprehensive as possible, striving to cover all health toxicological endpoints. If there is a data gap, additional explanations are generally required. The reason for doing this is that the results of these special toxicity tests will affect the selection of the most sensitive endpoint and the UF.
When calculating the AOEL or AREL, the most sensitive toxicity test endpoint no-observed-adverse-effect level (NOAEL) that matches the exposure duration and exposure route is generally divided by the UF. Taking the health risk assessment of applicators as an example, the exposure duration of applicators is generally short-term exposure, and the exposure route is mainly dermal and inhalation. Therefore, subacute or subchronic dermal and inhalation toxicity test data are generally used to derive dermal and inhalation AOEL/AREL. Pesticide oral subacute or subchronic toxicity data are more easily obtained, but can only be used as alternative data, and can only be used to derive dermal and inhalation AOEL/AREL when dermal or inhalation-related data are missing. When using subchronic toxicity test NOAEL to calculate the allowable exposure level, the UF generally defaults to 100 (ten times intra-species difference × ten times inter-species difference), and when using subacute toxicity test NOAEL, the UF is generally selected as 300.
When a pesticide has both dermal and oral subacute or subchronic toxicity data, do you have to choose dermal toxicity test data to calculate dermal AOEL/AREL? The answer is not necessarily, and the premise of doing so is that the dermal toxicity test data is reliable. In the dermal repeated exposure test, if the unit area administration dose is too large, the test substance may overflow and contaminate the hair of the test animal, and then the test animal may ingest the test substance orally through grooming behavior, showing oral-like toxic effects. In this case, using oral toxicity test data would be a more prudent choice.
So when using subacute and subchronic toxicity test NOAEL to derive AOEL/AREL), does the UF have to be 300 and 100, respectively? The answer is also not necessarily. For example, aerosol products generally prefer to use subacute toxicity test data to derive AOEL/AREL, in which case the UF is still 100. When a pesticide has significant special toxicity (such as mutagenicity) or the data quality is not very reliable, using UF=100 for protection is definitely not enough.
It is worth mentioning that there is a certain difference between China's method of calculating the AOEL/AREL of pesticides compared with that of the European Union and the United States, so the AOEL value in foreign assessment reports is generally not directly applicable to China's pesticide health risk assessment. The European Union EFSA assessment report generally calculates the AOEL of pesticides based on systemic toxicity, and it is not necessary to calculate the AOEL value for each exposure route separately. This health limit is based on the internal exposure dose (absorption dose), and most of it is calculated using oral toxicity test data. When the oral absorption rate is less than 80%, the actual oral absorption rate is used to correct the AOEL value. The allowable exposure levels provided in the US EPA public report are also mostly based on oral toxicity test data. However, China requires separate calculations for each exposure route and uses dermal toxicity data to calculate dermal AOEL/AREL. This value is essentially still based on the external exposure dose (administration dose) and generally does not require correction with the actual dermal absorption rate. When a pesticide lacks both dermal and inhalation subacute or subchronic toxicity data, the systemic allowable exposure level AOEL value in the European Union EFSA assessment report has great reference significance for China's pesticide health risk assessment.
Exposure Assessment
For exposure assessment, the exposure of operators is generally calculated using the international unit exposure method or assessment model.
For the health risk assessment of pesticide applicators, the exposure levels of inhalation and dermal routes are calculated using the following equation.
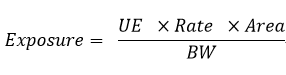
- Exposure: estimated exposure level, milligram/kilogram (mg/kg) body weight/density.
- UE: unit exposure, mg/kg, related to the formulation, crop, exposure route, and PPE.
- Rate: application rate per unit area, kilograms/hectare (kg/ha).
- Area: area applied per day, in hectares (ha), default is 1 ha.
- BW: body weight of operator, in kilograms (kg), default is 60.6kg.
Generally, the dermal and inhalation route exposure levels are calculated separately for the preparation and application of pesticides. They can be added to obtain the total exposure levels for dermal and inhalation routes. The biggest mistake in this step is to use dermal absorption rate data to optimize dermal exposure (using the dermal absorption rate of undiluted preparations for the preparation stage, and the dermal absorption rate of diluted formulations for the application stage) and then compare the total dermal exposure to the allowable dermal AOEL/AREL to calculate the dermal route risk quotient (RQ).
Why is this wrong? As mentioned earlier, the dermal allowable exposure levels based on dermal toxicity data (AOEL/AREL) are essentially based on external exposure dose (the administered dose), and can only be compared to the predicted external exposure levels. The dermal exposure levels optimized with the dermal absorption rate are internal exposure doses (absorbed doses) and they cannot be directly compared to AOEL/AREL.
When is it necessary to use dermal absorption rate to optimize dermal exposure? The answer is that when there is a lack of relevant dermal toxicity data, the AOEL/AREL of dermal toxicity is calculated based on alternative route data (such as oral or inhalation toxicity). Generally, the absorption rate of oral and inhalation routes is much higher than that of dermal routes. The AOEL/AREL values, which are based on internal exposure dose and corrected by the actual dermal absorption rate or default inhalation absorption rate (100%), are usually considered.
The current biggest challenge for exposure assessment is the lack of unit exposure data (UE), assessment models, and methods for specific application scenarios. For agricultural pesticides, the released unit exposure data only considers the spraying scenario for different crops and does not include multiple pesticide application scenarios such as seed treatment and granule applications. For public health pesticides, there is no assessment model for purposes other than mosquito coils, aerosols and repellents, such as fabric treatment and residual spraying.
When unit exposure data and related models are missing, companies can generally refer to international exposure parameters, models or health risk assessment methods, such as EFSA's pesticide exposure assessment guidance, the US EPA's Pesticide Handlers' Exposure Database (PHED) and EPA's SOP for Residential Exposure Assessment (SOP). When the tier 1 health risk assessment fails, high-tier exposure studies can also be conducted for specific pesticide application scenarios based on the guidelines for pesticide unit exposure testing, to obtain actual unit exposure levels.
Risk Characterization
Whether the operator health risk of pesticides is acceptable can be evaluated by using the RQ, which is the ratio of the estimated exposure level and calculated allowable exposure level. Generally, the RQ of dermal and inhalation exposure route is calculated separately, and then the comprehensive RQ is calculated by combining the RQ of these two routes. If the comprehensive RQ ≤ 1, the risk is acceptable; if the comprehensive RQ > 1, the risk is unacceptable.
Something that is easily overlooked in the risk characterization stage is to assess whether the toxic effects caused by each exposure route are the same (such as whether the target organ is the same). If different exposure routes cause different toxicity effects, the RQ of each exposure route should be used for risk characterization separately, instead of using the comprehensive RQ. For a formulation containing multiple active ingredients, the toxicity of each active ingredient should also be considered, and then the RQ of each active ingredient should be added to decide whether to sum up the RQs of each active ingredient.
Summary
This article summarizes the key technical points and precautions in the four main steps of hazard identification, hazard assessment, exposure assessment, and risk characterization in China's pesticide health risk assessment. The aim is to help technical personnel engaged in pesticide registration and toxicological risk assessment in the pesticide industry. China's pesticide health risk assessment methods have gradually started to align with international standards, but also have their own unique features. Although some pesticide products and application scenarios still lack health risk assessment methods and models, we feel optimistic about the improvement of pesticide health risk assessment in China in the future.
If you need any assistance or have any questions, please contact us via service@cirs-group.com.
Author

Yunbo Shi, Chief Technology Officer, Toxicologist, CIRS Group
Further Information

