In vitro methods are done outside of a living organism while in vivo methods are done on/in a living organism.
In health toxicology study, in vitro methods usually utilizes isolated organs, in vitro cultured bacteria, cells or organelles, and biological simulations in the laboratories to refine, reduce and replace traditional animal tests. In vitro methods are developed to protect animal welfare, which is a major focus in testing currently.
At present, in vitro methods for skin corrosion/irritation, eye irritation, and skin sensitization have been greatly developed, and have been applied in EU REACH registration. China is also advancing the application of in vitro methods step by step. For example, under the previous Measures for Environmental Management of New Chemical Substances (MEE Order No.7), positive results generated by in vitro skin corrosion/ irritation and eye irritation methods are accepted.
The Measures for Environmental Management Registration of New Chemical Substance (MEE Order No.12) entered into force on January 1st, 2021. Under the Guidance Documents for MEE Order No.12, minimum health toxicological data requirements shall cover skin corrosion/irritation data endpoint, eye irritation data endpoint, and skin sensitization data endpoint, either in vivo or in vitro is available. In vivo testing data is necessary when in vitro testing data is not available to conclude a result. This article summarizes the applicability of in vitro methods related to skin corrosion/irritation, eye irritation, and skin sensitization under MEE Order No.12.
In vitro skin corrosion/irritation method
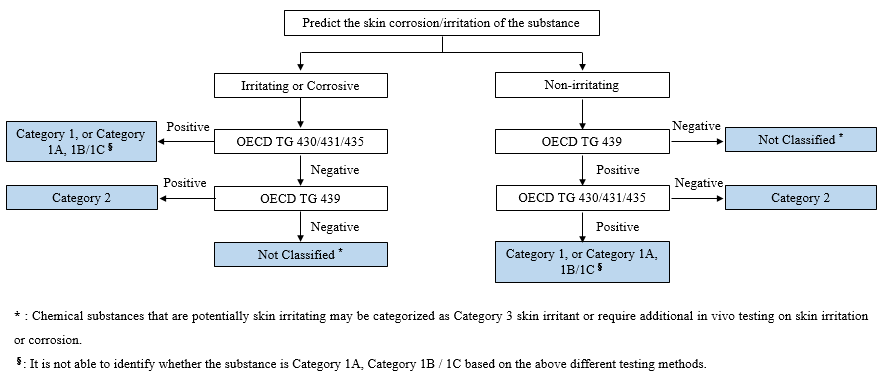
Two types of in vitro methods related to skin corrosion/irritation are commonly adopted: in vitro skin corrosion method (OECD TG 430/431/435) and in vitro skin irritation method (OECD TG 439). It is recommended that applicants for new chemical substances registration shall collect information to predict the potential corrosion and irritation of the chemical substances, and choose the appropriate methods based on the prediction.
In vitro eye irritation method
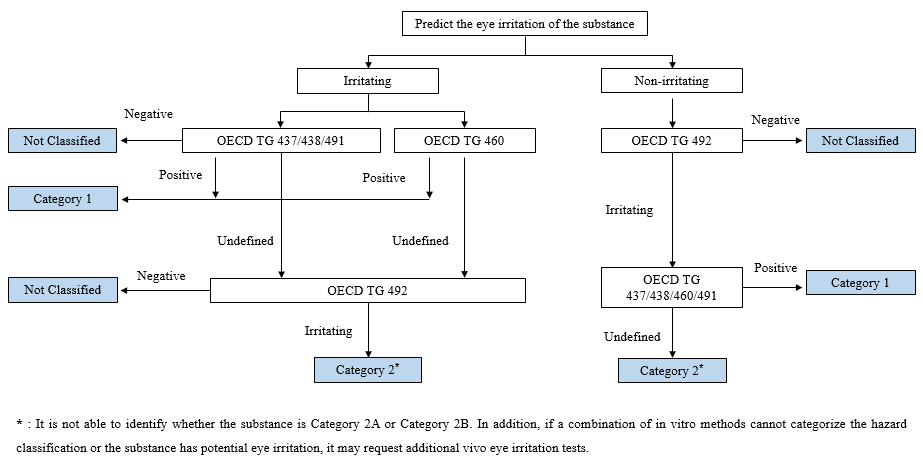
The commonly used in vitro eye irritation methods include OECD TG 437/438/460/491/492, which may vary in the scope of application, accuracy, and test results evaluation. To quick complete the hazard classification, it is necessary to select an appropriate combination of methods according to the predicted eye irritation of chemical substances.
In vitro skin sensitization method
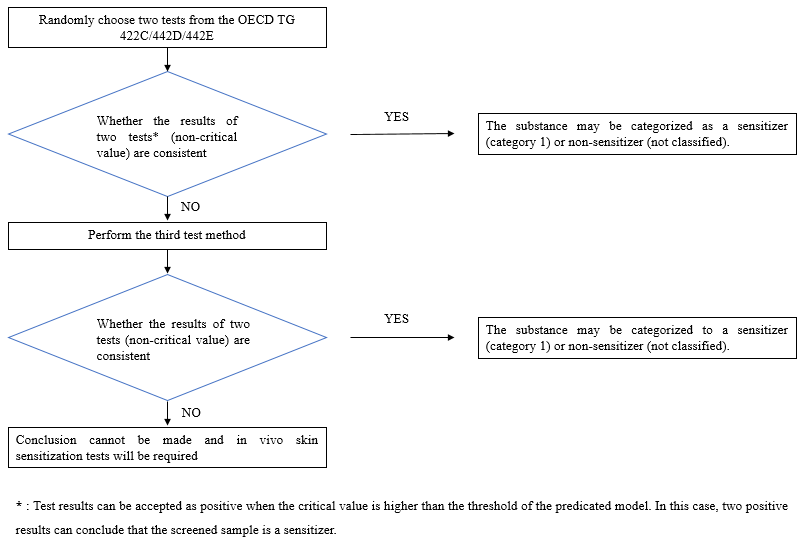
In vitro skin sensitization methods cover OECD TG 442C/442D/442E, which are widely adopted to address the three key events of the skin sensitization AOP and to comprehensively evaluate the potential skin sensitization hazards. Generally, enterprises just need to carry out two test methods out of OECD TG 442C/442D/442E to determine whether the substance is a sensitizer (Category 1) or non-sensitizer (not classified). A single key event is not sufficient to reach a conclusion that the substance has potential skin sensitization.
At present, only few laboratories are capable of performing in vitro methods on skin corrosion/irritation and eye irritation in China. Most of the in vitro skin sensitization methods are still under validation. Therefore, it is generally recommended to use the reports issued by foreign OECD GLP laboratories if in vitro method reports are requested. In addition, if the classification cannot be determined by the results arising from in vitro tests, it is necessary to perform the in vivo tests to fulfill the regulatory requirements.
Summary
This article summarizes the in vitro methods related to skin corrosion/ irritation, eye irritation, and skin sensitization that can be accepted under MEE Order No.12 and the recommended test strategies. In vitro methods have been widely accepted by the international community for purposes of protecting animal welfare and reducing the number of animals needed in testing. As more and more in vitro methods are being developed and improved with competent testing results, it is believed that a growing number of in vitro methods will be applied in the new chemical substance registration in China.
If you have any needs or questions, please contact us at service@cirs-group.com.

