According to the information released by the Center for Food Evaluation, State Administration for Market Regulation (SAMR), and the Special Food Information Query Platform, it issued a total of 2,130 health food (dietary supplement) registration approvals in 2025. Among them, 200 approvals were for new health food products, including 194 domestic products and six imported products.

CIRS conducted a detailed summary of these 200 new products, and analyzed from the following points.
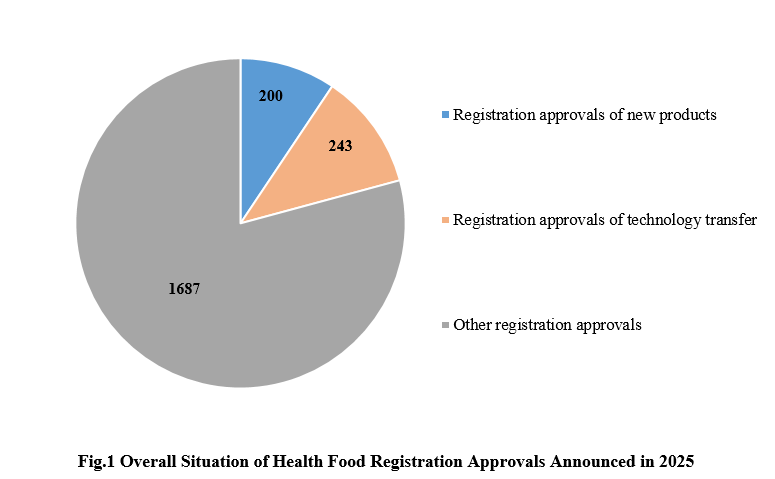
1. The Overall Situation of Health Food Registration Approvals Announced in 2025
Among the 2,130 registration approvals, 200 are new products, accounting for 9.39% of the total; 243 are products for technology transfer, accounting for 11.41% of the total. The other 1,687 registration approvals, not for new products, included renewal registration and change registration.
2. The Number of Approved Products in Different Countries/Regions
Among the 200 approved new products, domestic products are from 25 provinces (municipalities and/or autonomous regions). Beijing ranked first with 46 products, accounting for 23.71% of the total domestic new products. Imported products are from Hong Kong (four products), Indonesia (one product), and Singapore (one product).
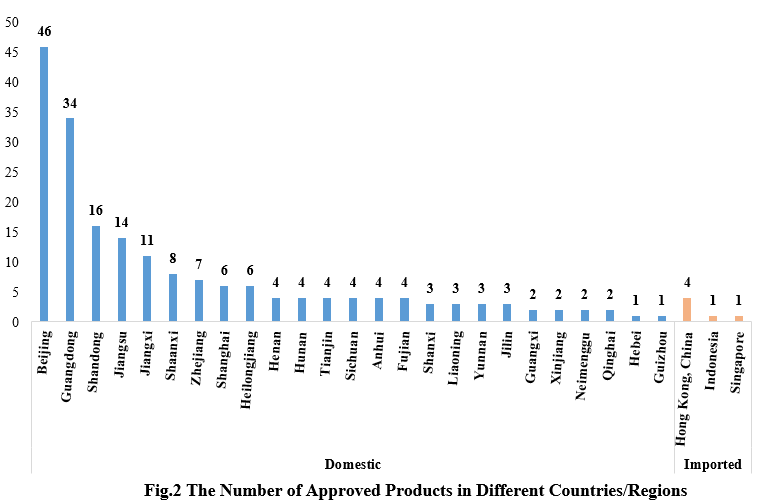
Note: If the applicant includes more than one enterprise and comes from different provinces, each province is included in the statistics.
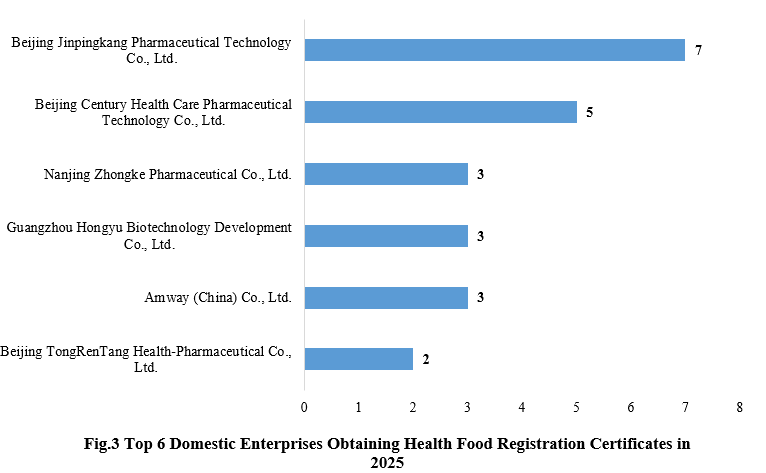
3. Enterprises Obtaining Health Food Registration Certificates
Beijing Jinpingkang Pharmaceutical Technology Co., Ltd. obtained seven new product registration approvals in 2025, taking the top position, followed closely by its parent company, Beijing Century Health Care Pharmaceutical Technology Co., Ltd., which obtained five new product registration approvals.
Of the six imported products, four were from WRIGHT LIFE PHARMACEUTICAL LIMITED, while the remaining two came from PT. HELMIGS PRIMA SEJAHTERA and HSIEHS BIOTECH, respectively. Refer to the table below for details.
Table1. Information for imported products approved in 2025
Product Name | Approval Number | Applicant | Country (region) of Production | Health Function |
WRIGHT LIFE NOTOGINSENG AND PANAX QUINQUEFOLIUS CAPSULES | 国食健注J20250001 | WRIGHT LIFE PHARMACEUTICAL LIMITED | Hong Kong, China | Aids in enhancing immunity |
HELMIG'S CURCUMIN TABLET | 国食健注J20250002 | PT. HELMIGS PRIMA SEJAHTERA | Indonesia | Aids in maintaining healthy blood lipid levels & provides auxiliary protective action against chemical liver damage |
WRIGHT LIFE GANODERMA SPORE CAPSULES | 国食健注J20250003 | WRIGHT LIFE PHARMACEUTICAL LIMITED | Hong Kong, China | Aids in enhancing immunity |
WRIGHT LIFE BLUEBERRY LUTEIN CAPSULES | 国食健注J20250004 | WRIGHT LIFE PHARMACEUTICAL LIMITED | Hong Kong, China | Alleviating visual fatigue |
WRIGHT LIFE AGARICUS BLAZEI GANODERMA CAPSULES | 国食健注J20250005 | WRIGHT LIFE PHARMACEUTICAL LIMITED | Hong Kong, China | Aids in enhancing immunity |
GOLDEN LYPRES | 国食健注J20250006 | HSIEHS BIOTECH | Singapore | Aids in enhancing immunity |
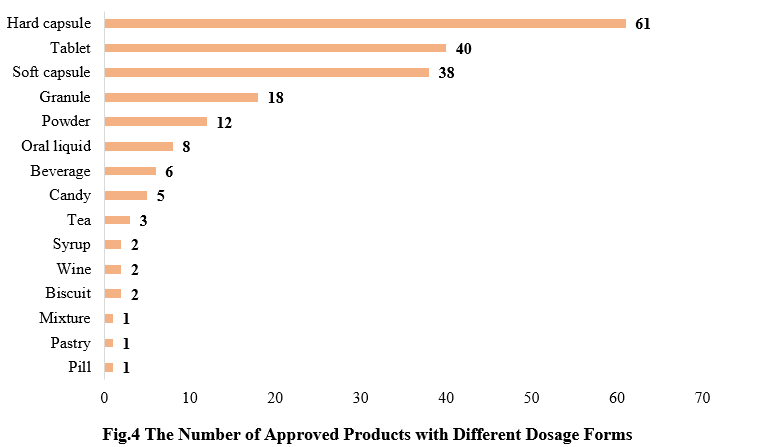
4. The Number of Approved Products with Different Dosage Forms
The dosage forms of approved new products (including imported products) includes: capsule, tablet, powder, and oral liquid. Among them, hard capsule products received the largest number of approvals (61), followed by tablet products (40).
5. The Number of Approved Products with Different Health Functions
5.1 Single health function
As shown in Fig.5-1, there are a total of 187 new products (including imported products) approved with a single health function. Among them, products with the health function of “Aids in enhancing immunity” received the largest number of approvals, with 71.
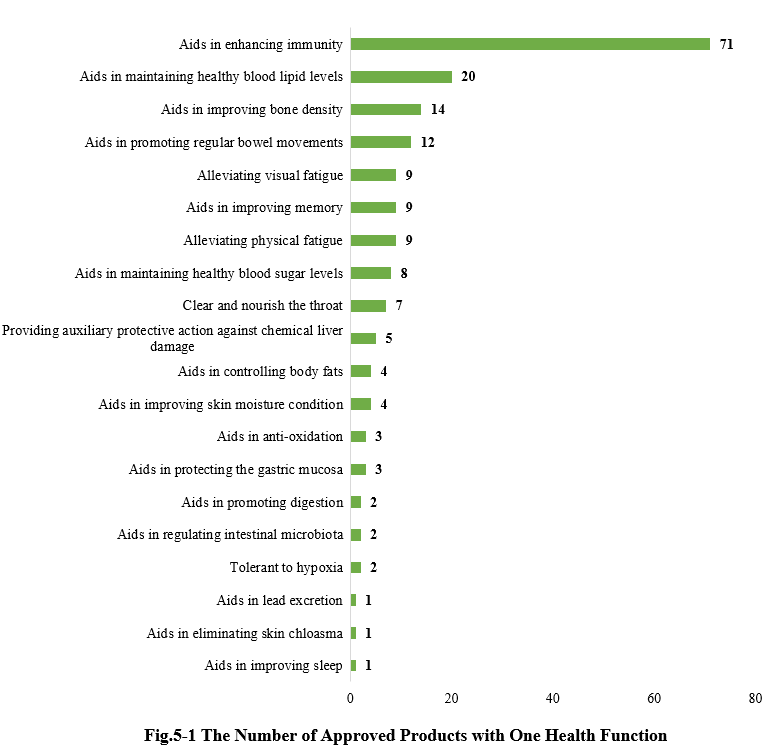
5.2 Two health functions
Except for those new products with a single health function, there are also 13 products approved with two health functions (shown in Fig.5-2).
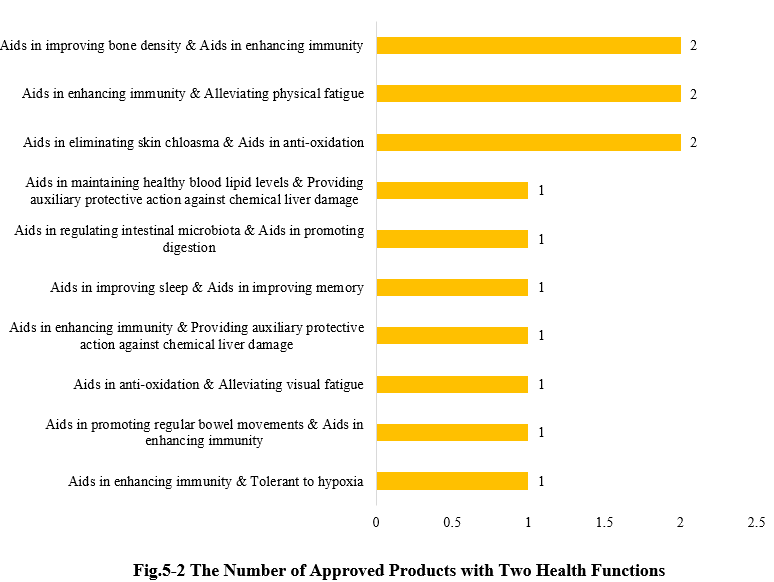
6. CIRS Comments
1) Fewer in quantity, but higher in quality
The stringent technical review and on-site inspection limit the number of approved new products to only a few hundred each year, thereby ensuring their safety and quality from the source. Unlike the health food filing products, thousands of which are approved each year, the registration products are fostering a “fewer but better” market model. Under this model, enterprises focus on health functions that are more targeted and closely aligned with societal health needs when developing new products. This approach enhances product value and competitiveness, enabling enterprises to command a premium even amid pricing pressure from filing products.
2) Imported products bring new vitality
The six imported products approved in 2025 broke the seven-year silence of "zero approval". This not only serves as a booster for foreign enterprises – signaling that China's health food market remains open to imported products that comply with regulatory requirements, but also serves as a reminder to domestic companies – with new imported products entering the market, competition will intensify, and the key to respond is to explore and strengthen differentiated advantages.
In 2025, GB 17405-1998 Good Manufacturing Practice for Health Food, which has been in force for over two decades, was updated for the first time. The new national standard will take effect on September 2, 2026. It refines requirements about indicator settings and management systems to better protect consumer health, foster industry development, and guide regulatory practice.
Note: There may be a lag in the data release of the Special Food Information Query Platform. The data in this article is only for reference, and the actual situation is subject to the official announcement.
Data Source:
Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

