Rhodiola is a traditional Chinese medicinal herb included in the Pharmacopoeia of the People’s Republic of China and is also listed in the Catalogue of Substances Permitted for Use in Health Foods. It is a commonly used raw material for health foods, primarily containing saponin compounds such as rhodioloside, which are associated with health functions including alleviating physical fatigue and improving tolerance to hypoxia. According to searches on official platforms, more than 500 registered health food products using Rhodiola or Rhodiola-derived products as raw materials have been approved to date.
Rhodiola has a wide range of applications and strong market demand in pharmaceuticals and health foods. However, precisely because of this demand, overharvesting has led to severe resource scarcity. To protect Rhodiola resources, the List of National Key Protected Wild Plants released on September 7, 2021, classified Rhodiola as a Class II protected species. This includes Rhodiola crenulata, the species commonly used in health foods in China, as well as Rhodiola rosea, which is widely used internationally.
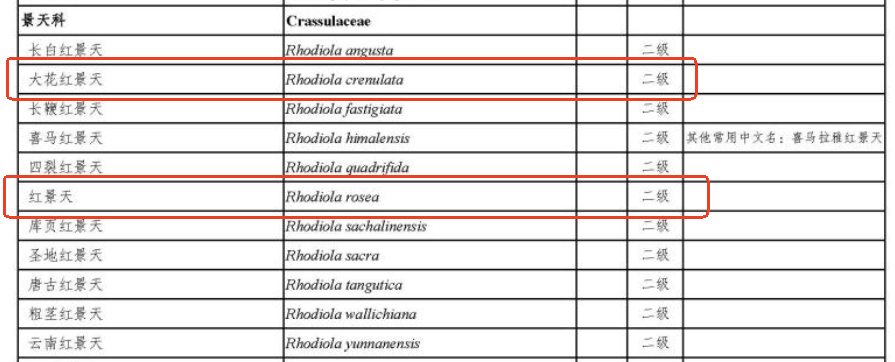
Figure 1. Rhodiola spp. in the List of National Key Protected Wild Plants
Meanwhile, all species of Rhodiola spp. have also been included in the Convention on International Trade in Endangered Species of Wild Fauna and Flora.
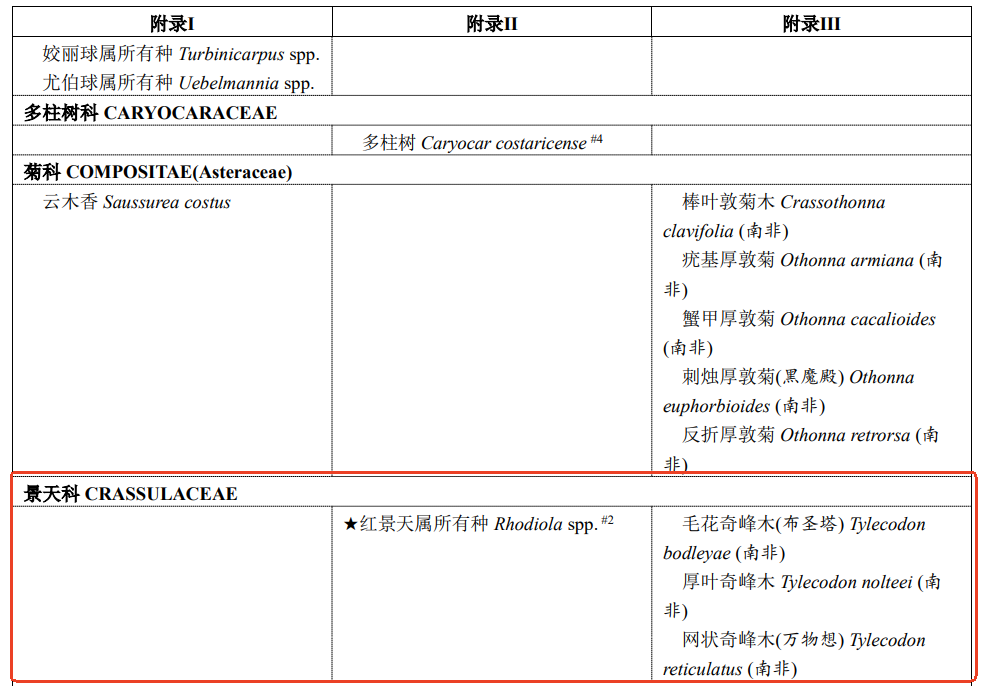
Figure 2. Rhodiola spp. in the Convention on International Trade in Endangered Species of Wild Fauna and Flora
According to the Provisions on the Application and Review of Health Foods Derived from Wild Animals and Plants (Trial), the use of national Class I and Class II protected wild animals and plants, as well as their products, as raw materials for health foods is prohibited in China. Artificially bred, propagated, or cultivated national Class I protected wild animals and plants are also prohibited from use. If artificially bred, propagated, or cultivated, national Class II protected wild animals and plants are used, supporting documents permitting their development and utilization must be provided.
In other words, companies may only source artificially cultivated Rhodiola for the development of health food products. Due to the scarcity of natural resources and strict protection measures, combined with the long growth cycle of Rhodiola and the technical difficulties associated with large-scale cultivation, CIRS Group has learned from multiple domestic and international enterprises that Rhodiola is currently extremely difficult to procure.
As resources continue to become increasingly scarce, the urgency of addressing the Rhodiola dilemma is growing. Looking ahead, in addition to strengthening conservation efforts and regulatory oversight, improving artificial cultivation technologies for Rhodiola represents a critical pathway forward.
However, relying solely on these measures may still be far from sufficient. Relevant enterprises can further explore innovative approaches in synthetic biology, using biotechnological fermentation to produce Rhodiola. This production method can significantly improve efficiency while reducing excessive exploitation of natural resources, thereby supporting green and sustainable development. The application of synthetic biology may become a key solution to the current resource constraints facing Rhodiola and could drive the green upgrading and transformation of the industry.
About the CIRS Group Food Team
Established in 2012, the Food Business Division of CIRS Group has helped over 1,000 food companies globally achieve one-stop compliance solutions. CIRS offers a full range of regulatory services covering novel food applications, synthetic biology-derived foods, U.S. GRAS notice, EU novel food application, health food registration, and food for special medical purposes (FSMP).
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

