As of December 31, 2022, China's State Administration for Market Regulation (SAMR) has issued a total of 1,445 health food and dietary supplement registration approvals, 421 of which were new health food products. We have conducted a detailed summary of these 421 new products and analyzed them from the following points.
1. Overall Situation of Health Food Registration Approvals Announced in 2022
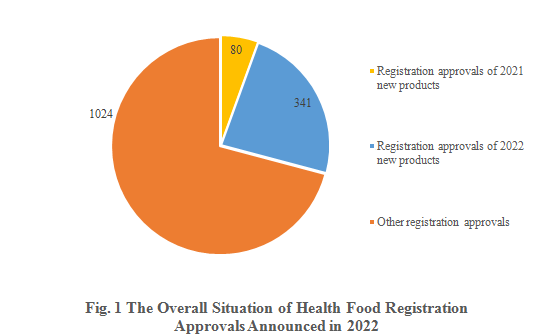
Among the 1,445 registration approvals, 421 are new products, accounting for 29.13% of the total. Of all the new products, 80 were approved in 2021 and 341 are approved in 2022. The other 1,024 registration approvals were registration renewals, alterations, and transfer of technology registrations.
2. The Number of Health Food Registration Approvals Announced in Each Month
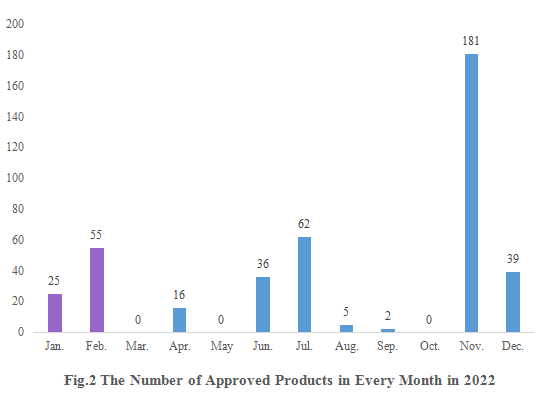
As shown in Fig.2, registration approvals of 2021 new products are announced in January and February (shown in purple). And that of 2022 new products were not announced until April (shown in blue).
3. The Number of Approved Products in Different Regions
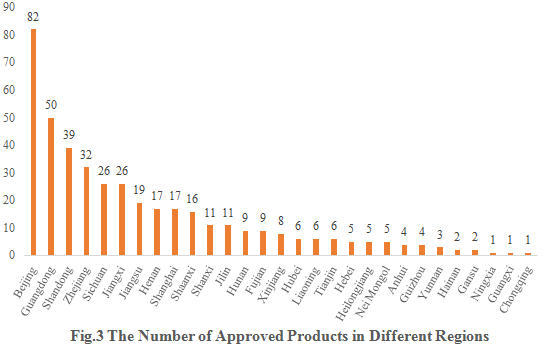
The 421 approved new products are from 29 provinces (including municipalities and/or autonomous regions). Beijing, Guangdong, and Shandong occupy the top three spots with 82, 50 and 39 respectively, accounting for 19.48%, 11.88%, and 9.26% of the total new products.
4. Enterprises Obtaining Health Food Registration Certificates
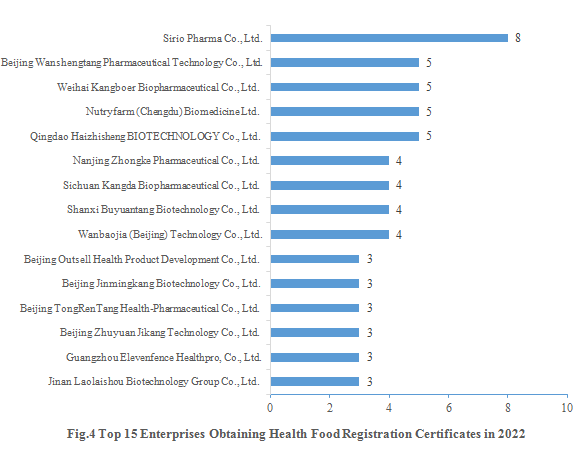
Sirio Pharma Co., Ltd. gained eight new registration certificates in 2022, followed by Beijing Wanshengtang Pharmaceutical Technology Co., Ltd., Weihai Kangboer Biopharmaceutical Co., Ltd., Nutryfarm (Chengdu) Biomedicine Ltd. and Qingdao Haizhisheng BIOTECHNOLOGY Co., Ltd., each of which gained five new registration certificates. The top 15 enterprises obtaining new registration certificates in 2022 are shown in the Fig.4.
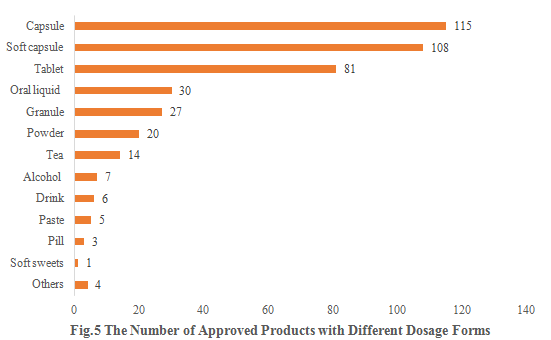
5. The Number of Approved Products with Different Dosage Forms
Among these approved new products, capsules, soft capsules, and tablets remain the three most popular dosage forms, with the products quantity of 115, 108, and 81, accounting for 27.32%, 25.65%, and 19.24%, respectively.
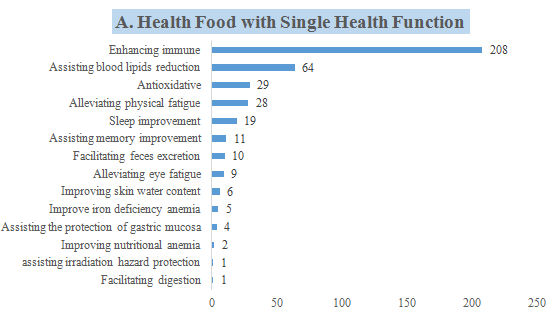
6. The Number of Approved Products with Different Health Functions
CIRS collected statistics of the corresponding health functions of these 421 approved new products, and conducted the following analysis.
- 397 products with a single health function, which is mainly enhancing the immune system, with a total of 208 products, accounting for 52.39% of the 397 products.
- 23 products with two health functions, the health function combination of “enhancing the immune system and alleviating physical fatigue” is the most popular, with 12 products, accounting for 52.17% of the 23 products.
- Only one product declared the health function as “supply vitamin/mineral”, and the specific health function is “supply calcium, magnesium, vitamin D and vitamin K”.

7. CIRS Comments
The total number of approved health food new products in 2022 is higher than that of 2021. Throughout the health food registered product market, since the health food function evaluation method is not officially published, the overall situation is still a bit lacking. Unlike filing products with strict limits for health functions, registered products have a wider range of declaration so that they can better meet the health needs of different groups.
Meanwhile, the SAMR issued the Detailed Rules for Technical Evaluation of New Functions of Health Food (Trial) (Drafts) in August providing detailed guidance and specifications for health food enterprises to register for new functions and new products, which not only promotes products innovation but also helps the market renew. With the continuous improvement to health food policies, it can be believed that the registered health food will gradually increase.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Data Source
Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.
Note
There may be a lag in the data release of the Special Food Information Query Platform. The data in this article is only for reference, and the actual situation is subject to the official announcement.

