As of December 31, 2021, according to the Special Food Information Query Platform of the State Administration for Market Regulation (SAMR), 112 health food (dietary supplement) products got the registration approval in China in the year of 2021, including 110 new products and 2 products for technology transfer.
In order to help enterprises have an overview of this kind of products in China, CIRS counted the data of the approved health food registration products in 2021, and made an analysis for your reference.
1. The Overall Situation of Health Food Registered Approval Issued in 2021
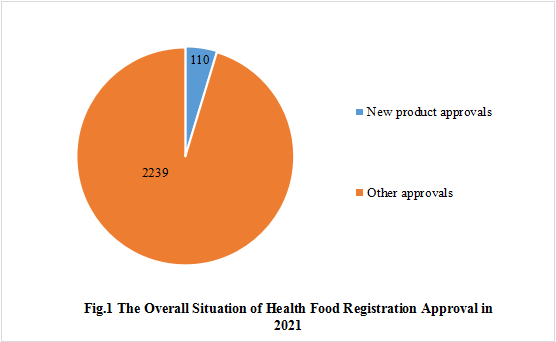
In fact, the Food Evaluation Center of the SAMR issued the registration approval information of 2349 health food products in 2021. Among them, only 110 of them are newly registered products, and the other 2239 products are approved for registration renewal, registration alteration, technology transfer, etc.
2. The Number of Health Food Approved for Registration in Every Month
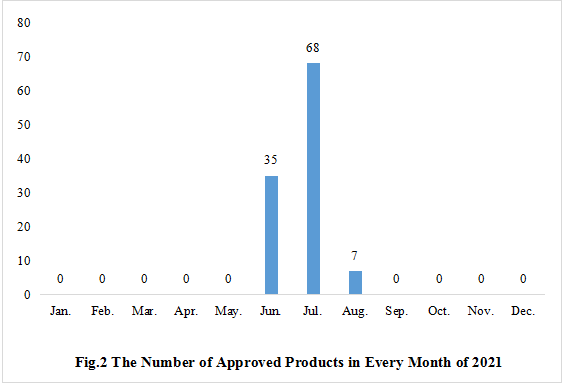
It can be seen in Fig.2, the issuance of new product registration approvals is mainly concentrated between June and August. (The issuance of other approvals is mainly concentrated between January and June, as well as between September and December, not showed in the Fig)
3. The Number of Approved Products in Different Regions
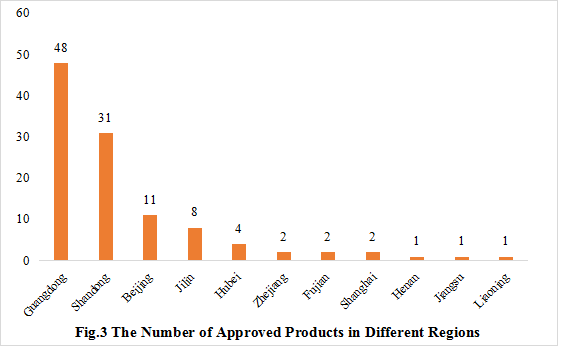
The 110 approved new products are from 11 provinces and/or municipalities. Guangdong Province has been the most, with the number of 48, accounting for 43.64% of the total, followed by Shandong Province, with the number of 31, accounting for 28.18% of the total.
4. Enterprises Obtaining Health Food Registration Certificates
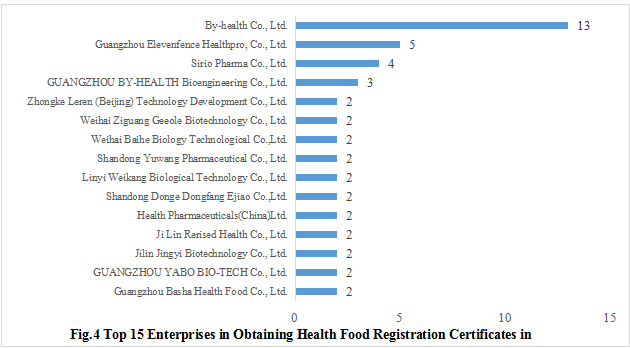
The 110 approved new products come from 76 health food enterprises, of which By-health Co., Ltd. has got the largest number of registration certificates (13), followed by Guangzhou Elevenfence Healthpro, Co., Ltd. with the number of 5. Figure 4 shows the top 15 enterprises with the specific number of approved registration certificates in 2021.
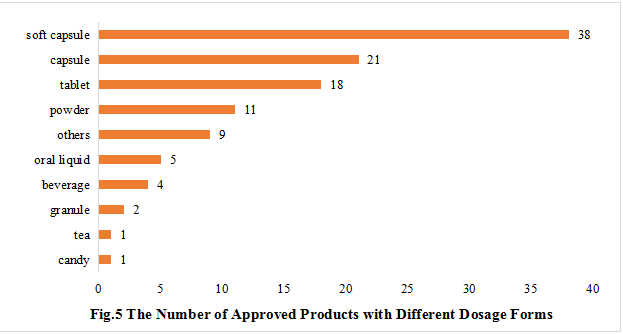
5. The Number of Approved Products with Different Dosage Forms
The dosage forms of approved new products include capsule, soft capsule, tablet, powder, oral liquid, granule, beverage, tea, candy, etc., and are mainly soft capsules, with the number of 38, accounting for 34.55% of the total, followed by capsules, tablets and powder with the number of 21, 18 and 11, accounting for 19.09%, 16.36% and 10.00% of the total, respectively.
6. The Number of Approved Products with Different Health Functions
The health functions of the 110 approved new products have been all published. The details are analyzed as follows:
1)105 products with single health function, which is mainly enhancing immune, with the number of 50, accounting for 47.62% of the 105 products.
2)4 products with two health functions, which are regulating gastrointestinal tract flora and facilitating feces excretion, assisting blood lipids reduction and enhancing immune, enhancing immune and alleviating physical fatigue, respectively.
3) 1 product with the functions of supplementing vitamins/minerals, which is supplementing Calcium and Vitamin D.
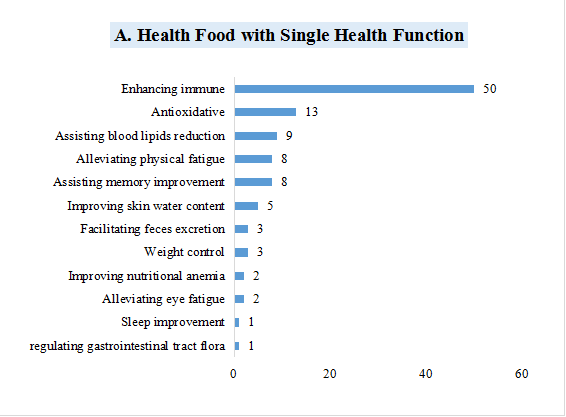
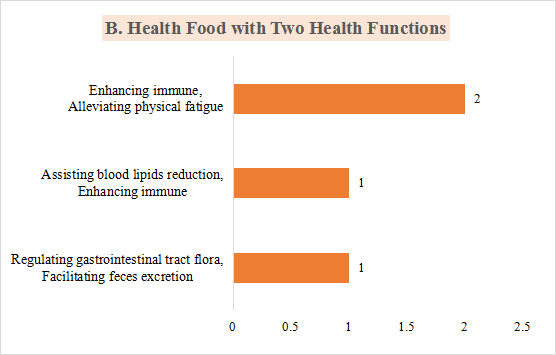
Fig.6 The Number of Approved Products with Different Health Functions
7. CIRS Comments
The number of newly registered products in 2021 is lower than that in 2020 (The number of products registered in 2020 was 715). It is estimated that most of products stranded in previous years were reviewed in 2020, and the official version of the function evaluation methods was still not released yet, which hinders the new products application in recent years. At the beginning of 2022, it can be expected that the function evaluation methods will be issued quickly, so that enterprises can carry out the new products registration application smoothly.
Click to view the statistics and analysis on China Health Food (Dietary Supplement) registration products in 2020
If you have any needs or questions, please contact us at service@cirs-group.com.
Data Source
Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.

