In 2017, CFDA conducted monthly inspection on domestic infant formula milk powder. A total of 2657 batches of domestic infant formula milk powder are tested, 8 batches of them are unqualified. The pass rate is 99.7%.
In 2017, 35 batches of imported infant
formula milk powder are returned or destroyed at port of entry.
The main reasons for the denial are “out of shelf life” and “submitting
incompliant certificates or supporting documents”.
Domestic Infant Formula Milk Powder Inspection Result in 2017
1. Pass Rate
In 2017, CFDA carried out monthly
inspection on domestic infant formula milk powder, and the inspection result
was released on CFDA website every month. According to CIRS statistics, a total
of 2657 batches of domestic infant formula milk powder are tested, 8 batches of
them are unqualified. The pass rate is 99.7%.
2. Unqualified Items
8 batched of products are unqualified mainly due to: enterobacter
sakazakii contamination; inconsistence between nutrition test value
and label value; imcompliant label. Details are as following:
Month | Unqualified Batch | Reasons for Disqualification |
February | 1 | Enterobacter sakazakii is detected. |
March | 4 |
|
April | 1 | Enterobacter sakazakii is detected. |
May | 2 | The labeling of vitamin B12, folic acid and biotin in the nutrition information is not compliant with GB 13432, which stipulates that the nutrition value should be the specific number and labeling format like "value range" and "the maximum and/or minimum " is not allowed. |
Imported Infant Formula Milk Powder Inspection Result at the Port in 2017
1. Basic Inspection Result
In 2017, a total of 35 infant formula milk powder were returned or destroyed at port of entry. They are from 8 manufacturers of 4 countries, involving 15 brands.
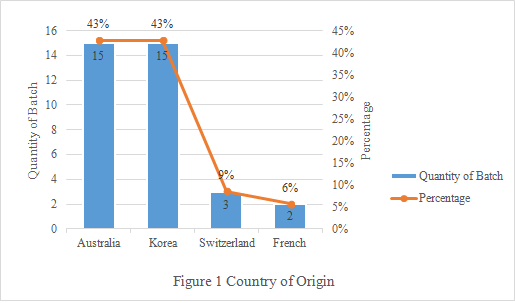
2. Country of Origin
The rejected infant formula milk powder are mainly from Australia and Korea, accounting for 86% in total (Seen Figure 1 below). For Australia, the main reason for rejection is “submitting incompliant certificates”. While for Korea, the only reason is “out of shelf life”.
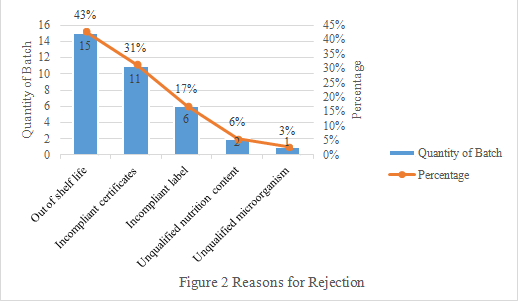
3. Reasons for Rejection
The main reasons for rejection are out of shelf life and submitting incompliant certificates, accounting for 74% in total (Seen Figure 2 below).
CIRS Suggestions
According to 2018 Food Safety Inspection Plan, CFDA will continue to conduct monthly inspection on domestic infant formula milk powder this year. Therefore, the relevant domestic enterprises should always take strict supervision on the production of infant formula milk powder, not only to ensure the product quality and safety, but also to help establish a good corporate image.
For imported infant formula milk powder, the product quality and product label should meet the requirements of Chinese regulations. Besides, related enterprises are suggested to import products with fresh production date to China, so that the products won't easily exceed the expiry date when importing. And they should double confirm the list of the required certificates and documents as well, so that all the materials can be well-prepared before importing.
If you have any needs or questions, please contact us at service@cirs-group.com.

