Since 2016, China has formally proposed to carry out the "three reductions and three health" action, advocating that citizens could eat less salt, less oil, and less sugar and maintain oral health, weight health and bones health”.
To encourage "sugar reduction", several government functional departments have released related policy documents, these include:
- On June 24, 2019, the State Council issued the Opinions on the Implementation of the Healthy China Action, and the Healthy China Action (2019-2030) at the national level, which clearly proposing the strategic goal of "sugar reduction”.
- The National Health Commission of the People’s Republic of China also released the Health Oral Action Plan (2019-2025), further proposing the goal of “sugar reduction”.
- China Beverage Health Consumption Forum released the White Paper on Sugar Reduction Action for Healthy China's Beverage and Food (2021), which advocates "sugar reduction" in beverages and food.
1. Implementing the "sugar reduction" policy from food raw materials
With the healthy lifestyle deeply rooted in the hearts of citizens, "sugar reduction" is widely accepted by citizens and becomes a trend of global food production and consumption. "Sugar reduction" refers to reducing the intake of added sugar or free sugar (sugar in natural fruits and natural carbohydrates in staple foods excluded). Added sugar refers to the sugar added to food artificially, which has sweet taste, including monosaccharides and disaccharides. Common added sugars cover sucrose, fructose, glucose, etc. Therefore, it is an ideal way to reduce the use of added sugar in food by taking sweet substances that are not absorbed by the human body or have low utilization rate as the source of sweetener. In recent years, more and more food raw materials with sugar substitutes have been developed and applied, and the following are some popular ones:
1) L-arabinose
The sweetness of L-arabinose is 50% - 60% of sucrose and it tastes pure and soft. The digestibility and absorption rate of L-arabinose in the small intestine is low. After entering the large intestine, L-arabinose is fermented to short chain fatty acids by microorganisms, so that its intake will not lead to an increase in blood sugar. Clinical trials have shown that L-arabinose can block the metabolism and transformation of sucrose, and reduce the metabolism and absorption of sucrose by inhibiting the enzymes that hydrolyze disaccharides.
Compliance status: At present, L-arabinose has been approved as a new food raw material in China.
- The original Ministry of Health approved L-arabinose (from corn cob, corn husk and other gramineous plant fibers) as a new resource food in Announcement No. 12 in 2008, and the scope of application is all kinds of food except for baby food.
- Since then, the National Health Commission of China has terminated the review of two L-arabinose applications, and the review conclusion is "substantially equivalent". One of them has the consistent source with the approved published L-arabinose, and the other is from gum arabic.
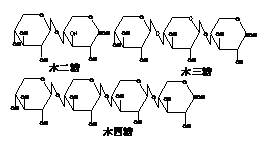
2) Xylo-oligosaccharides
Xylo-oligosaccharides is the oligosaccharides mixture linked by β-1,4 glycosidic bonds, and it tastes similar to sucrose with the sweetness 50% of sucrose. Since there is no enzyme that hydrolyzes β-1,4glycosidic bond in human digestive system, xylo-oligosaccharides can’t be digested and absorbed by human body. The xylo-oligosaccharides ingested by the human body are used by beneficial bacteria in the large and small intestine, which will not lead to increased blood sugar and fat deposition. Xylo-oligosaccharides in diet can not only meet people's demand for sweet taste, but also help maintain human health.
Compliance status: Xylo-oligosaccharides have also been approved as new food raw materials in China. The original Ministry of Health approved Xylo-oligosaccharides as new resource food in Announcement No. 12, 2008. The relevant information is as follows:
Name | Xylo-oligosaccharides |
Main ingredient | Xylobiose-xylheptatose |
Source | Wheat straw |
Structural formula |
|
Brief description of production process | Produced from wheat straw by steam explosion and xylanase hydrolysis. |
Consumption | ≤1.2g/day(calculated by xylobiose-xylheptatose) |
Scope of use | All kinds of food except baby food |
3) D-psicose
The sweet taste of D-psicose is 70% of sucrose while its calories are only about 10% of sucrose. Moreover, D-psicose has a taste similar to sucrose, so it’s an ideal low calorie sucrose substitute. When D-psicose is ingested into the human body, 70% will be directly discharged with urine or feces. D-psicose inhibits fatty liver enzymes and intestinal tract α- Glycosidase, which can reduce the small intestine absorption of carbohydrates to inhibit the increase of blood sugar and reduce the accumulation of body fat.
Compliance status: D-psicose has not been approved in China, but the enthusiasm of the manufacturer to declare is very high. Since 2020, the National Health Commission of China has published the acceptance information of D-psicose on its official website for three times. In foreign countries, D-psicose has been used in food to a certain extent. In terms of compliance, take Europe and the United States for example:
- The US Food and Drug Administration (FDA) has issued four GRAS certification announcements (generally recognized as safe substances) for D-psicose, which are intended to be used as sugar substitutes in some food categories;
- The EU has not yet approved D-psicose, but many enterprises have submitted applications to the European Food Safety Administration (EFSA) for D-psicose as a new food raw material. At present, these applications are in the risk assessment status.
4) D-tagatose
The sweet taste of D-tagatose is similar to sucrose and its sweetness is 92% of sucrose. It has no bad smell and aftertaste. Its calories is only 1/3 of that of sucrose, therefore, it is also an ideal substitute for sucrose. The human body does not have the tagatose-6-phosphate pathway to catabolism D-tagatose, so the absorption rate of D-tagatose in the small intestine is very low. The part not absorbed by the small intestine reaches the large intestine and is completely fermented by microorganisms to generate short chain fatty acids. D-tagatose can also inhibit the absorption of glucose in the small intestine. What’s more, some studies have also shown that there is no significant change in blood sugar and insulin levels after ingesting D-tagatose.
Compliance status: D-tagatose is compliant in China. The China National Health and Family Planning Commission approved D-tagatose as a new food raw material in Announcement No. 10 in 2014. The relevant information is as follows:
Name | Source | Brief description of production process | Scope of use |
D-tagatose | Galactose | With galactose as raw material and producing by steps of isomerization, decolorization, desalination, concentration, crystallization, etc. | Except for baby food. |
2. Sugar substitutes in food additives
In addition to the food raw materials with sugar substitute function, sweeteners in food additives are widely used as sugar substitutes. At present, there are about 20 sweeteners approved for use in China, such as aspartame, acesulfame, saccharin, cyclamate, sucralose, erythritol and stevioside. They are low in calories, similar in taste to sucrose yet tens or hundreds of times sweeter than sucrose. Food manufacturers and operators can also use some sweeteners instead of added sugar to achieve the goal of "sugar reduction". However, the use of sweeteners shall comply with the requirements of GB 2760-2014 on the amount and scope of use. Moreover, if the expected scope and amount of use can’t meet the requirements of GB 2760-2014, enterprises need to report to the National Health Commission of China in advance to expand the scope or amount of use of additives.
Epilogue
Under the national call of "sugar reduction", more and more substances with the role of sugar substitute emerge and be constantly applied. Many raw materials will not have a negative impact on health factors such as blood sugar and weight while meeting consumers' demand for sweet taste of various foods. Food manufacturers and operators can also use these raw materials to develop and innovate various and distinctive sugar-reduced food, which plays an important role in developing food industry and enriching people's material life.
At present, many raw materials with sugar substitutes have been approved in China and they can be used legally as well, however, there are also some new raw materials/additives that have been continuously developed. According to the relevant provisions of the Food Safety Law of China, the production of food with new food raw materials or the production of new varieties of food additives needs to pass the safety assessment of the National Health Commission of China. With professional technology, multiple resources and global network, the food regulation business department of CIRS Group provides customers with:
- Registration and declaration of new food raw materials
- Declaration service for new varieties of food additives
If you have any needs or questions, please contact us at service@cirs-group.com.
To help enterprises understand the industry's dynamics and clarify the direction of declaration. CIRS Group will hold a webinar on October 20 to introduce the correlation and difference between "new food raw materials" and "new food additives", and talk about the latest progress of hot ingredients such as "breast milk oligosaccharide (HMOs), β-nicotinamide single nucleotide (NMN)".
Main contents
- Regulatory background;
- How to distinguish an ingredient suitable for the application of new food raw material or new food additive?
- What are the key points of the application? What are the similarities and differences?
- How to determine the direction of application for ingredients produced by transgenic technology?
- Industry new trend, hot ingredients such as HMOs, NMN compliance progress