In 2023, the National Health Commission of the People’s Republic of China (NHC) issued five announcements (No. 1 of 2023, No. 3 of 2023, No. 5 of 2023, No. 8 of 2023, and No. 10 of 2023) regarding “Three New Food”. A total of 74 products, including new food raw materials (novel food), new food additives, and food-related products (new food contact substances) have been approved, 12 of which are new food raw materials (novel food), namely Leuconostoc pseudomesenteroides, blueberry anthocyanins, rye pollen, yellowhorn seed kernel, yellowhorn leaf, peach gum, tiger nut, Leuconostoc mesenteroides subsp. Cremoris, pyrroloquinoline quinone disodium (PQQ) salt, catechins, yerba mate, and yeast protein.

Related Links
Analysis on the Application and Approval of New Food Additives in 2023
Furthermore, according to the official announcements issued by the NHC and the China National Center for Food Safety Risk Assessment (CFSA), throughout 2023:
- 22 new food raw materials applications were accepted;
- 17 new food raw materials were issued for public comment;
- 4 products were rejected for approval; and
- 5 substances were included in the termination review list.
CIRS has summarized the acceptance and approval status of new food raw materials in China in 2023 as follows:
1. List of accepted new food raw materials in 2023 (22 types)
In 2023, NHC accepted the applications for 22 new food raw materials, with the majority being domestically produced products and a minority being imported products. The detailed technical review status is listed in the table below:
S.N. | Date of acceptance | Acceptance code | Name | Technical review status |
1 | 2023.02.02 | 卫食新申字(2023)第0001号 | Pyrroloquinoline quinone disodium (PQQ) salt | 2023.10.07 Approved |
2 | 2023.02.15 | 卫食新申字(2023)第0002号 | Pleurotus eryngii mycelium | 2023.06.20 Rejected for approval |
3 | 2023.03.09 | 卫食新申字(2023)第0003号 | Bacillus subtilis | 2023.08.28 Issued for public comments |
4 | 2023.03.24 | 卫食新申字(2023)第0004号 | Fermented mycelial proteins of Fusarium venenatum strain TB01 | 2023.06.20 Rejected for approval |
5 | 2023.04.06 | 卫食新申字(2023)第0005号 | N-Acetylglucosamine | Not issued for public comments yet |
6 | 2023.05.10 | 卫食新申字(2023)第0006号 | Pyrroloquinoline quinone disodium (PQQ) salt | Not issued for public comments yet |
7 | 2023.07.10 | 卫食新申字(2023)第0007号 | Bifidobacterium animalis subsp. Lactis XLTG11 | Not issued for public comments yet |
8 | 2023.07.19 | 卫食新申字(2023)第0008号 | Stevia rebaudiana | Terminated the review |
9 | 2023.07.25 | 卫食新申字(2023)第0009号 | D-psicose | Not issued for public comments yet |
10 | 2023.07.25 | 卫食新进申字(2023)第0001号 | Green oat extract | 2023-10-07 Rejected for approval |
11 | 2023.08.28 | 卫食新申字(2023)第0010号 | Drumstick Dendrobium flower | Not issued for public comments yet |
12 | 2023.08.28 | 卫食新申字(2023)第0011号 | Egg Yolk Lecithin; Egg Phospholipids | Not issued for public comments yet |
13 | 2023.09.04 | 卫食新申字(2023)第0012号 | Theanine | Not issued for public comments yet |
14 | 2023.09.04 | 卫食新申字(2023)第0013号 | Nannochloropsis gaditana Extract | Not issued for public comments yet |
15 | 2023.09.06 | 卫食新申字(2023)第0014号 | L-alpha-Glycerylphosphorylcholine | 2023.12.22 Issued for public comments |
16 | 2023.09.07 | 卫食新申字(2023)第0015号 | Stevia rebaudiana Extract | Not issued for public comments yet |
17 | 2023.09.11 | 卫食新进申字(2023)第0002号 | Leuconostoc pseudomesenteroides | 2023.12.22 Issued for public comments |
18 | 2023.09.12 | 卫食新申字(2023)第0016号 | Golden tea flowers | Terminated the review |
19 | 2023.09.28 | 卫食新进申字(2023)第0003号 | Corn fermentation | Not issued for public comments yet |
20 | 2023.10.23 | 卫食新申字(2023)第0017号 | Malania oleifera oil | Not issued for public comments yet |
21 | 2023.11.16 | 卫食新进申字(2023)第0004号 | Bifidobacterium longum biovar infantis M-63 | Not issued for public comments yet |
22 | 2023.11.22 | 卫食新进申字(2023)第0005号 | Oat green extract | Not issued for public comments yet |
2. List of new food raw materials issued for public comments in 2023 (17 types)
In 2023, 17 new food raw materials have passed the technical review of CFSA and were issued for public comments. (Only the 6 raw materials that are still in the public consultation stage and have not been approved yet are presented below. See Part 3 for detailed information on the other 11 approved raw materials including blueberry anthocyanins.).
Details are as follows:
(1) Eggshell membrane extract (Draft announcement)
Name | Eggshell membrane extract |
Basic information | Source: Eggshell membrane |
Brief introduction of the production process | Prepared through eggshell-membrane separation, sterilization, hydrolysis, mixing, drying, grinding, and other processes by using eggshell as the raw material. |
Recommended intake | ≤500 mg/day |
Other information | 1. The labels and instructions shall bear a statement that they should not be consumed by infants, pregnant women, lactating women and people with an allergy to eggs, along with the recommended intake. 2. Quality specifications and food safety indicators are detailed in the draft announcement. |
Acceptance date | Accepted on September 17, 2021. The acceptance code is 卫食新进申字(2021)第0006号. Acceptance name: 蛋壳膜粉 |
Issued date | Passed the technical review and issued for public comments on January 12, 2023 |
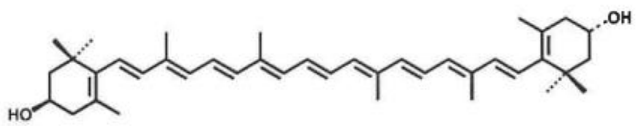
(2) (3R,3'S)-β,β-carotene-3,3'-Diol (Draft announcement)
Name | (3R,3'S)-β,β-carotene-3,3'-Diol |
Basic information | Source: Tagetes Erecta L. CAS number: 31272-50-1 Molecular formula: C40H56O2 Molecular weight: 568.88 Structure:
|
Brief introduction of the production process | Prepared through dehydration, crushing, extraction, isomerization, purification, drying processes, and other processes by using Tagetes Erecta L as the raw material. |
Recommended intake | ≤8 mg/day (count as (3R,3'S)-β,β-carotene-3,3'-Diol) |
Other information | Application scope and maximum levels: Milk and dairy products (10 mg/kg); beverages: 160 mg/kg for liquid beverages ≤50 mL, 16 mg/kg for liquid beverages of 51-500 mL; solid beverages are converted according to the mass of liquid after preparation; bakery food (20 mg/kg); candy (3g/kg); ready-to-eat cereals (15 mg/kg); frozen beverages (30 mg/kg), excluding infants and young children food. Food safety indicators shall meet the requirements as outlined in the draft announcement. |
Acceptance date | Accepted on March 17, 2022. The acceptance code is 卫食新申字(2022)第0002号. |
Issued date | Passed the technical review and issued for public comments on August 28, 2023. |
(3) Pichia kluyver (Draft announcement)
Name | Pichia kluyver |
Other information | Being included in the List of Strains that Can Be Used in Food and approved for use in the fermentation processing of fermented wine, fruit and vegetable juices, and tea drinks (excluding infants and young children foods). The labels and instructions shall bear a statement of the scope of use. Food safety indicators shall meet the requirements as outlined in the draft announcement. |
Acceptance date | Accepted on December 5, 2022. The acceptance code is 食新进申字(2022)第0006号. |
Issued date | Passed the technical review and issued for public comments on August 28, 2023. |
(4) Bacillus subtilis DE111 (Draft announcement)
Name | Bacillus subtilis DE111 |
Other information |
|
Acceptance date | Accepted on March 9, 2023. The acceptance code is卫食新申字(2023)第0003号. Acceptance name: 枯草芽孢杆菌 |
Issued date | Passed the technical review and issued for public comments on August 28, 2023. |
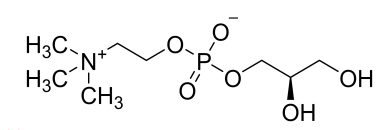
(5) L-alpha-Glycerylphosphorylcholine (Draft announcement)
Name | L-alpha-Glycerylphosphorylcholine |
Basic information |
CAS number: 28319-77-9 Molecular formula: C8H20NO6P Relative molecular mass: 257.22 |
Brief introduction of the production process | Prepared through condensation and esterification reactions, followed by decolorization, impurity removal, concentration, refinement, drying and other processes using polyphosphoric acid, choline chloride, (R)-(-)-3-Chloro-1,2-propanediol, sodium hydroxide and water as raw materials. |
Recommended intake | ≤ 600 mg/day (on a dry basis) |
Other information | The labels and instructions shall bear a statement that they should not be consumed by infants, pregnant women, and lactating women, along with the recommended intake. Quality specifications and food safety indicators are detailed in the draft announcement. |
Acceptance date | Accepted on September 6, 2023. The acceptance code is 卫食新申字(2023)第0014号. |
Issued date | Passed the technical review and issued for public comments on December 22, 2023. |
(6) Leuconostoc pseudomesenteroides (Draft announcement)
Name | Leuconostoc pseudomesenteroides |
Other information | 1. Included in the List of Strains that Can Be Used in Food. The applicable scope includes fermented milk, flavored fermented milk, cheese, fermentation milk drinks and lactobacillus drinks (non-solid drinks), cream, butter and anhydrous milkfat, excluding infants and young children food. 2. Food safety indicators shall meet the requirements as detailed in the draft announcement. |
Acceptance date | Accepted on September 11, 2023. The acceptance code is 卫食新进申字(2023)第0002号. Acceptance name: 假肠膜明串珠菌 |
Issued date | Passed the technical review and issued for public comments on December 22, 2023. |
3. List of approved new food raw materials in 2023 (12 types)
There were 12 new food raw materials approved in 2023, including 2 strains that can be used in food, namely Leuconostoc pseudomesenteroides and Leuconostoc mesenteroides subsp. cremoris. The detailed approval information for each product is as follows:
(1) Leuconostoc pseudomesenteroides
Name | Leuconostoc pseudomesenteroides |
Other information | Included in the List of Strains that Can Be Used in Food. The applicable scope includes fermented milk, flavored fermented milk, cheese, fermentation milk drinks and lactobacillus drinks (non-solid drinks), excluding infants and young children's food. Food safety indicators shall meet the relevant requirements as detailed in the announcement. |
Acceptance date | Accepted on April 19, 2021. The acceptance code is 卫食新进申字(2021)第0002号. Acceptance name: 假肠膜明串珠菌 |
Issued date | Passed the technical review and issued for public comments on June 29, 2022. |
Approved date | Officially approved on March 2, 2023 according to Notice No.1, 2023. |
(2) Blueberry anthocyanins
Name | Blueberry anthocyanins |
Basic information | Source: Blueberry derived from Vaccinium corymbosum L. |
Brief introduction of the production process | Prepared through enzymolysis, water extraction, purification, concentration, drying and other processes, using blueberries as raw materials. |
Recommended intake | ≤800 mg/day |
Other information | Applicable scope and maximum usage level: Milk and dairy products (modified milk and flavored fermented milk: 0.8 g/kg; formulated milk powder shall be converted according to the liquid volume after preparation; cheese, process(ed) cheese, cheese products and condensed milk shall be converted according to the raw milk usage amount); beverages (0.8 g/kg; solid beverages shall be converted according to the liquid volume after preparation); jelly (14 g/kg), cocoa products, chocolates and chocolate products (including chocolate and chocolate products with cocoa butter substitute) (14 g/kg ); candy (40 g/kg); frozen beverages (8 g/kg); bakery foods (4 g/kg); alcoholic beverages (4 g/kg). The labels and instructions shall bear a statement that they should not be consumed by infants, pregnant women, and lactating women, along with the recommended intake. Food safety indicators shall meet the relevant requirements as detailed in the announcement. |
Acceptance date | Accepted on October 26, 2021. The acceptance code is 卫食新申字(2021)第0012号. Acceptance name: 蓝美1号蓝莓提取物 |
Issued date | Passed the technical review and issued for public comments on January 12, 2023. |
Approved date | Officially approved on May 6, 2023 according to Notice No. 3, 2023. |
(3) Rye pollen
Name | Rye pollen |
Basic information | Source: Secale Cereale L. |
Brief introduction of the production process | Prepared through pollen collection, drying, separation and other processes by using rye as the raw material. |
Recommended intake | ≤1.5 g/day |
Other information | The labels and instructions shall bear a statement that they should not be consumed by infants, pregnant women and lactating women, along with the recommended intake. Food safety indicators shall meet the relevant requirements as detailed in the announcement. |
Acceptance date | Accepted on October 25, 2021. The acceptance code is 卫食新进申字(2021)第0008号. |
Issued date | Passed the technical review and issued for public comments on January 12, 2023. |
Approved date | Officially approved on May 6, 2023 according to Notice No. 3, 2023. |
(4) Yellowhorn seed kernel
Name | Yellowhorn seed kernel |
Basic information | Source: Seeds of Xanthoceras sorbifolium Bunge |
Brief introduction of the production process | Prepared through drying, magnetic separation, dehulling, screening, and other processes by using the seeds of Xanthoceras sorbifolium Bunge as raw materials. |
Other information | The labels and instructions shall bear a statement that they should not be consumed by infants, pregnant women, and lactating women, along with the recommended intake. Refer to the announcement for detailed quality requirements. Food safety indicators shall comply with the current national food safety standards for nuts and seeds. |
Acceptance date | Accepted on March 29, 2021. The acceptance code is 卫食新申字(2021)第0006号. |
Issued date | Passed the technical review and issued for public comments on March 10, 2023. |
Approved date | Officially approved on August 1, 2023 according to Notice No. 5, 2023. |
(5) Yellowhorn leaf
Name | Yellowhorn leaf |
Basic information | Source: Young leaves of Xanthoceras sorbifolium Bunge |
Brief introduction of the production process | Prepared through fixation, rolling, drying, and other processes by using the young leaves of Xanthoceras sorbifolium Bunge as raw materials. |
Recommended intake | ≤6 g/day |
Other information | The labels and instructions shall bear a statement that they should not be consumed by infants, pregnant women, and lactating women, along with the recommended intake. Edible method: brewing Food safety indicators shall meet the relevant requirements as detailed in the announcement. |
Acceptance date | Accepted on June 6, 2022. The acceptance code is 卫食新申字(2022)第0006号. |
Issued date | Passed the technical review and issued for public comments on March 10, 2023. |
Approved date | Officially approved on August 1, 2023 according to Notice No. 5, 2023 |
(6) Peach gum
Name | Peach gum |
Basic information | Source: Prunus persica (L.) Batsch |
Brief introduction of the production process | Prepared through picking, sorting, airing, cleaning, drying, and other processes by using the gum secreted from Prunus persica (L.) Batsch. |
Recommended intake | ≤30 g/day |
Other information | The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women, and lactating women, along with the recommended intake. Food safety indicators shall meet the relevant requirements as detailed in the announcement. |
Acceptance date | Accepted on May 7, 2022. The acceptance code is 卫食新申字(2022)第0004号. |
Issued date | Passed the technical review and issued for public comments on April 23, 2023. |
Approved date | Officially approved on October 7, 2023 according to Notice No. 8, 2023 |
(7) Tiger nut
Name | Tiger nut |
Basic information | Source: Cyperus esculentus L. var. sativus Boeck. Edible part: stem tubers |
Other information | Food safety indicators shall comply with the current national food safety standard for nuts and seeds. Refer to the announcement for detailed quality specifications. |
Acceptance date | Accepted on September 5, 2022. The acceptance code is 卫食新申字(2022)第0010号. |
Issued date | Passed the technical review and issued for public comments on April 23, 2023. |
Approved date | Officially approved on October 7, 2023 according to Notice No. 8, 2023. |
(8) Leuconostoc mesenteroides subsp. cremoris
Name | Leuconostoc mesenteroides subsp. cremoris |
Other information |
|
Acceptance date | Accepted on November 23, 2021. The acceptance code is 卫食新申字(2021)第0014号。 |
Issued date | Passed the technical review and issued for public comments on April 23, 2023. |
Approved date | Officially approved on October 7, 2023 according to Notice No. 8, 2023. |
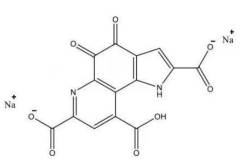
(9) Pyrroloquinoline quinone disodium (PQQ) salt
Name | Pyrroloquinoline quinone disodium (PQQ) salt |
Basic information | CAS: 122628-50-6 Molecular formula: C14H4N2Na2O8 Molecular weight: 374.17 Structure:
|
Brief introduction of the production process | Prepared through fermentation, extraction, purification, crystallization, drying, and other processes by using Methylovorus glucosotrophus as the production strain. |
Recommended intake | ≤20 mg/day |
Other information |
|
Acceptance date | Accepted on February 2, 2023. The acceptance code is 卫食新申字(2023)第0001号. |
Issued date | Passed the technical review and issued for public comments on April 23, 2023. |
Approved date | Officially approved on October 7, 2023 according to Notice No. 8, 2023. |
(10) Catechins
Name | Catechins |
Basic information | Source: Camellia sinensis (L.) O. Ktze. |
Brief introduction of the production process | Prepared through alcohol extraction, concentration, separation, extraction, enzymolysis, concentration, drying, and other processes using tea leaves as raw materials. |
Recommended intake | ≤300 mg/day (count as the total catechins) |
Other information | Applicable scope and maximum usage level: beverages (0.6 g/kg, solid beverages shall be converted according to the mass of liquid after preparation); candy (0.2 g/kg). The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women, and lactating women, along with the recommended intake. When being used and consumed simultaneously with EGCG as specified in Notice No.17 of 2010, the recommended total intake is set at ≤300 mg/day (counted as the total catechins). Quality specifications and food safety indicators are detailed in the announcement. |
Acceptance date | Accepted on October 27. The acceptance code is 卫食新申字(2021)第0013号. Acceptance name: 微晶复合儿茶素 |
Issued date | Passed the technical review and issued for public comments on January 12, 2023. |
Approved date | Officially approved on December 1, 2023 according to Notice No.10, 2023. |
(11) Yerba mate
Name | Yerba mate |
Basic information | Source: Leaves of Ilex paraguariensis A.St.-Hil. |
Brief introduction of the production process | Prepared through picking, roasting, chopping, drying, and other processes using leaves of Ilex paraguariensis A.St.-Hil. as raw materials. |
Other information | The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women, and lactating women, along with the recommended intake. Edible method: brewing. Food safety indicators shall meet the requirements as specified in the draft announcement. |
Acceptance date | Accepted on November 2, 2022. The acceptance code is 卫食新进申字(2022)第0004号. Acceptance name: 马黛茶叶 |
Issued date | Passed the technical review and issued for public comments on June 21, 2023. |
Approved date | Officially approved on December 1, 2023 according to Notice No.10, 2023. |
(12) Yeast protein
Name | Yeast protein |
Main component | Yeast protein |
Brief introduction of the production process | The bacterial raw material is obtained through breeding, fermenting, and centrifuging using Saccharomyces Cerevisiae as the strain basis. It is then prepared through nucleic acid removal, centrifugation enzymolysis, extraction, purification, separation, sterilization, drying, and other processes. |
Other information | The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women, and lactating women. Food safety indicators shall meet the relevant requirements as specified in the announcement. |
Acceptance date | Accepted on July 26, 2022. The acceptance code is 卫食新申字(2022)第0008号. |
Issued date | Passed the technical review and issued for public comments on March 10, 2023. |
Approved date | Officially approved on December 1, 2023 according to Notice No.10, 2023. |
4. Raw materials included in the List of Termination of Review (5 types)
In 2023, 5 raw materials were included in the list of termination reviews. Currently there are 73 products on the list.
No. | Acceptance code | Name |
1 | 卫食新申字(2022)第0007号 | |
2 | 卫食新进申字(2021)第0009号 | |
3 | 卫食新申字(2021)第0015号 | |
4 | 卫食新申字(2023)第0008号 | |
5 | 卫食新申字(2023)第0016号 |
5. Raw materials rejected for approval in 2023 (4 types)
In 2023, 4 products were rejected for approval by NHC including two microorganism fermented protein products (domestic) accepted in 2023, one plant extract product (imported), and Periploca sepium Bunge leaves (domestic) accepted in 2022. Details are as follows:
No. | Acceptance date | Acceptance code | Name | Review process/conclusion |
|---|---|---|---|---|
1 | 2022.08.11 | 卫食新申字(2022)第0009号 | Periploca sepium Bunge leaves | 2022.10.18 Delivered a notification of review opinion on the new food raw material; 2023.02.07 Issued a decision of not granting administrative permission. |
2 | 2023.02.15 | 卫食新申字(2023)第0002号 | Pleurotus eryngii mycelium | 2023.04.17 Delivered a notification of review opinion on the new food raw material; 2023.06.20 Issued a decision of not granting administrative permission. |
3 | 2023.03.24 | 卫食新申字(2023)第0004号 | Fermented mycelial proteins of Fusarium venenatum strain TB01 | 2023.04.17 Delivered a notification of review opinion on the new food raw material; 2023.06.20 Issued a decision of not granting administrative permission. |
4 | 2023.07.26 | 卫食新进申字(2023)第0001号 | Green Oats extract | 2023.06.20 Delivered a notification of review opinion on the new food raw material; 2023.10.07 Issued a decision of not granting administrative permission. |
6. Conclusion
It can be observed through the analysis of the application cycle of this year that, for the majority of raw materials, the process from being accepted to collecting public comments can be completed within one year, and for some, the cycle can be shortened to six months. Among the 12 approved new food raw materials, three-quarters of them can complete the whole process within two years. It can be seen that most enterprises have made sufficient preparations during the application process. However, there have also been cases of some raw materials not being approved. Therefore, it is crucial for enterprises intending to apply to conduct a comprehensive feasibility analysis at the beginning and make full preparations for documentation. This not only facilitates the relevant departments in advancing the work and shortening the review cycle, while also allowing companies to save time and costs.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Note: There may be omissions and errors in the data and statistics. The data in this article is for reference only, please refer to the official information published by government departments.
 Structure:
Structure: