In recent years, an increasing number of domestic and international companies have sought the FDA GRAS notice for new substances added to food. The U.S. Food and Drug Administration (FDA) regularly updates the list of "Generally Recognized as Safe (GRAS)" substances on its official website, with the most recent update as of October 31, 2023.
CIRS Group has made a detailed analysis and summarized the substances submitted for GRAS notice from 2021 to 2023 to provide businesses with valuable insights.
Summary of FDA GRAS notice from 2021-2023
Over the past three years, 231 products have been submitted for the FDA GRAS notification. Among them, 117 were approved, while 31 ceased evaluation as requested (based on cessation dates: 13 in 2021, 14 in 2022, and 4 in 2023). The remaining 83 are still pending after submission.

Figure 1. Overview of GRAS notifications from 2021 to 2023
Substances notified from 2021 to 2023 (based on approval dates): a total of 117 substances
The number of substances obtaining GRAS notice is declining from 2021 to 2023, possibly due to the increasing necessity for extensive and comprehensive data to support the GRAS conclusion for a growing number of new substances. Moreover, the surge in submission quantities has also led to delays in the review process.
It's noteworthy that Chinese enterprises successfully secured notification for 8 substances during this period, including:
- 3 types of steviol glycosides,
- 2 types of DHA algae oils,
- 1 functional substance D-psicose,
- 1 protein, and
- 1 enzyme preparation.
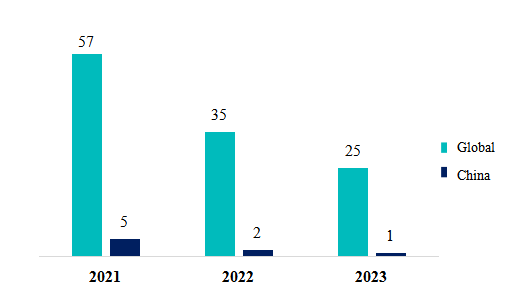
Figure 2. Number of notified FDA GRAS substances both domestically and globally from 2021-2023
Table 1. FDA GRAS notice obtained by Chinese enterprises from 2021-2023
No. | GRN No. | Substance | Notified date | Enterprise |
1 | 1029 | D-psicose | 2023/8/4 | L&P Foods Technology Co., Ltd. |
2 | 1021 | Transglutaminase enzyme preparation produced by Streptomyces mobaraensis strain M2020197 | 2022/11/3 | Dongsheng Technology Co., Ltd. |
3 | 1008 | Algal oil (≥45% docosahexaenoic acid) from Aurantiochytrium limacinum TKD-1 | 2022/2/25 | Anhui ATK Biotech Co., Ltd. |
4 | 999 | Enzyme-modified steviol glycosides | 2021/12/3 | Zhucheng Howtian Co., Ltd. |
5 | 983 | Purified steviol glycosides from the leaves of Stevia rebaudiana (Bertoni) | 2021/6/28 | Zhucheng Howtian Co., Ltd. |
6 | 970 | Enzyme-modified steviol glycosides | 2021/4/9 | Shandong SYX Stevia Biotech Co., Ltd. |
7 | 948 | Enzyme-treated pea protein | 2021/9/9 | Yantai Oriental Protein Technology Co., Ltd. |
8 | 934 | Algal oil (≥35% docosahexanoic acid (DHA)) derived from Schizochytrium sp. strain CABIO-A-2 | 2021/7/20 | Cabio Biotech (Wuhan) Co., Ltd. |
Substances in pending status (based on submission dates) from 2021 to 2023: a total of 83 substances
As illustrated in Figure 3, 2022 witnessed the most significant number of pending substances, totaling a substantial 51 substances. It's worth noticing that the FDA has extended the time for archiving new submissions (granting GRN No.) due to the surge in submissions. Consequently, many products submitted in 2023 are either yet to be archived or updated on the official website.
Regarding Chinese enterprises, among the 7 pending substances, there are 2 functional ingredients (GPC and PQQ), 2 unsaturated fatty acid supplements (fungal oil and algae oil), 1 probiotic, 1 anti-bacterial agent, and 1 antioxidant.
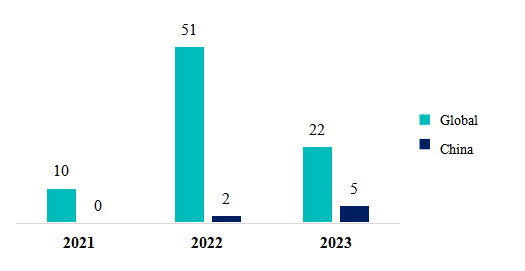
Figure 3. Substances in pending status both domestically and globally from 2021-2023
Table 2. Substances in pending status submitted by Chinese enterprises (published) from 2021-2023
No. | GRN No. | Substance | Enterprise |
1 | 1141 | L-α-glycerylphosphorylcholine (GPC) | Shenyang Gold Jyouki Technology Co.,Ltd. |
2 | 1138 | Hydroxytyrosol | Hangzhou Viablife BoitechCo.,Ltd. |
3 | 1134 | Bacteriophage preparation specific to Salmonella Enteritidis | Qingdao PhagepharmBoitech Co.,Ltd. |
4 | 1130 | Lactobacillus rhamnosus CGMCC21225 | FenghuaBoitech Co.,Ltd |
5 | 1128 | Algal oil (≥35% docosahexaenoic acid) from Schizochytrium sp. | Jiangsu Grand Xianle Pharmaceutical Co.Ltd. |
6 | 1118 | Pyrroloquinoline quinone disodium(PQQ)salt | Zhejiang Medicine Co., Ltd. |
7 | 1115 | Fungal oil (≥40% arachidonic acid (ARA)) from Mortierella alpina strain AF | Hubei Fuxing BoitechCo.,Ltd. |
Popular GRAS substances from 2021 to 2023
Over the past three years, popular GRAS substances can be categorized into several categories, including:
- 24 HMOs,
- 8 steviol glycosides,
- 54 strains, and
- 29 enzyme preparations.
HMOs and steviol glycosides have seen a substantial number of notifications, aligning with the rapid advancement in synthetic biotechnology in recent years. Additionally, there is a wide variety of enzyme preparations, including amylases, proteases, invertase, and complex enzymes.
Other GRAS products serve various functions, including processing aids (emulsifiers and anti-bacterial agents), nutritional raw materials (protein, milk powder, algae oil, plant extracts, and oligosaccharides), and functional ingredients (D-psicose, dihydroquercetin and L-carnitine), etc.
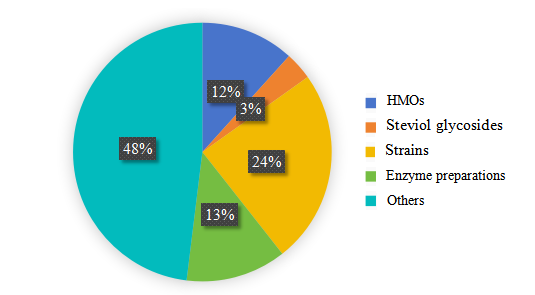
Figure 4. Overview of hot substances notifications from 2021-2023
Table 3. Details of hot substances from 2021-2023
Category | Substance | Number of notifications | Number of pending substances | Total |
HMOs | 2'-FL | 5 | 3 | 8 |
3-FL | 3 | 1 | 4 | |
3'-SL | 3 | 0 | 3 | |
6'-SL | 4 | 0 | 4 | |
LNT | 3 | 0 | 3 | |
LNnT | 2 | 0 | 2 | |
Total | 20 | 4 | 24 | |
Steviol glycosides | Rebaudioside B | 1 | 0 | 1 |
Rebaudioside I | 2 | 0 | 2 | |
Rebaudioside M | 1 | 0 | 1 | |
Mixed monomers | 3 | 1 | 4 | |
Total | 7 | 1 | 8 | |
Strains | Brewer’s yeast | 4 | 1 | 5 |
Bifidobacterium | 8 | 7 | 15 | |
Lactobacillus | 4 | 10 | 14 | |
Bacillus subtilis | 6 | 3 | 9 | |
Others | 2 | 9 | 11 | |
Total | 24 | 30 | 54 |
*Data Source: The inventory of GRAS notices and the latest released accepted GRAS dossiers (updated as of 2023/10/31).
*Note:
1. As the FDA doesn’t disclose dossier acceptance dates, the statistics for substances in pending status are primarily based on the submission dates recorded in the released dossiers.
2. The statistics are based on the published dossiers with GRN No. and are for reference only.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

