Under Cosmetics Supervision and Administration Regulations (CSAR) issued in 2020, cosmetics are divided into special cosmetics and ordinary cosmetics. Special cosmetics are products for the function of anti-freckle/whitening, sunscreen, hair dye, hair perm, anti-hair loss, and new efficacies. Ordinary cosmetics are cosmetics other than special cosmetics. Companies who plan to place cosmetics on the Chinese market must apply for registration or filing with the NMPA or provincial MPA to get approval license or filing certificate. Toothpaste will be regulated as ordinary cosmetics.
Qualification of Cosmetics Registrant and Filer
The cosmetics registrant or filer is in charge of the quality, safety, and efficacy claims of cosmetics. And it shall
Be an enterprise or other organization established according to law;
Have a quality management system suitable for the cosmetics to be registered and filed;
Have an ability to adverse reactions monitoring and evaluation
Duty of Domestic Responsible Person
Foreign companies have to appoint a domestic responsible person to deal with the pre-market registration or filing application. The domestic responsible person shall
Register and file the cosmetics or new cosmetic ingredients in the name of the registrant and filer;
Assist registrants and filers in the monitoring of cosmetics adverse reactions, safety monitoring, and reporting of new cosmetic raw materials;
Assist registrants and filers in the recall of cosmetics and cosmetic ingredients;
Undertake the corresponding safety and quality responsibilities of cosmetics and new cosmetic ingredients placed in the Chinese market according to the agreement between the responsible person and registrant/filer;
Cooperate with the supervision and inspection work of the supervision departments
Who Shall Register?
Companies who intend to sell cosmetics products via offline stores in China
Companies who intend to sell cosmetics products via online platforms except cross-border e-commerce such as TMALL, JD, etc.,
Application Types
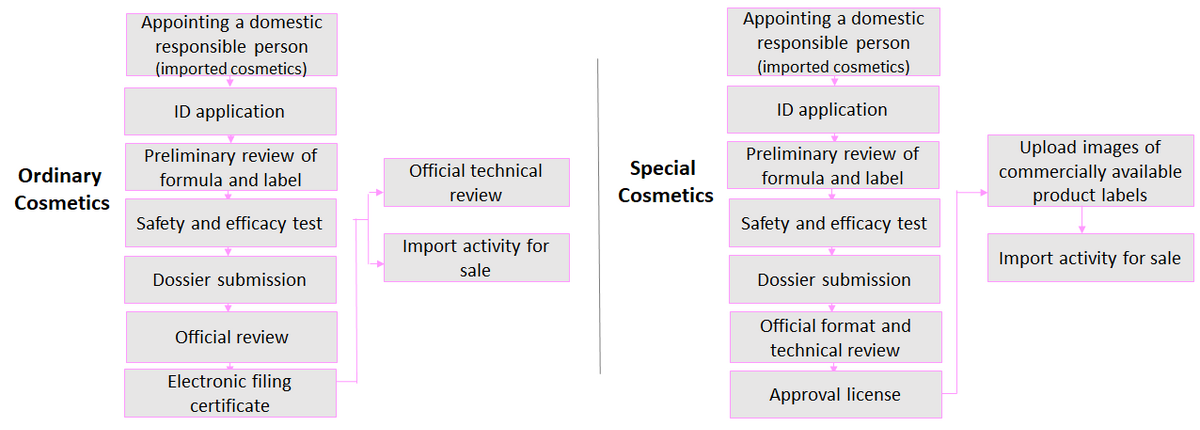
There are two types of applications for cosmetics under CSAR: registration and filing:
Registration of special cosmetics |
|
|---|---|
Filing of ordinary cosmetics |
|
Application Process
Our Services
Registration of Special |
|
|---|
Further Information