The year 2020 is a new regulatory year for the cosmetics and toothpaste industry. With the implementation of the Cosmetics Supervision and Administration Regulations (CSAR), NMPA has issued a series of draft supporting regulations. Although it is a new era to the supervision of cosmetics and toothpaste, the registration of new cosmetics in 2020 is still proceeding steadily.
1. Registration and filing of cosmetics
1.1 Special use cosmetics
In 2020, there are still 9 categories of special cosmetics. Nearly 2,800 domestic special use cosmetics have been registered, with the highest proportion still being sunscreen and anti-freckle products, which are more than 500 and 1000 SKU respectively, among which about 170 products have both the effects of sunscreen and anti-freckle. The total number of imported special use cosmetics is about 1400 SKU, and there are no bodybuilding and breast shaping products, as shown in Figure 1.
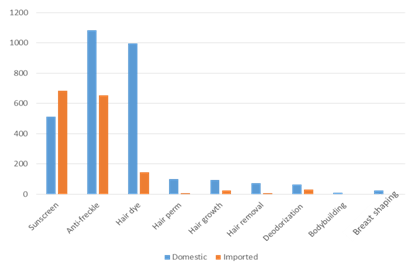
Figure 1 Statistics of the number of 9 categories of registered special use cosmetics in 2020 *
* Note: The number of sunscreen and anti-freckle cosmetics includes the number of products with both the effects of sunscreen and anti-freckle
Though the cosmetics for hair growth, hair removal, breast shaping, bodybuilding and deodorization in 2020 are still approved, but the Article 78 of Cosmetics Supervision and Administration Regulations clearly points out that "the cosmetics registered before the implementation of the CSAR for hair growth, hair removal, breast shaping, bodybuilding and deodorization will be given with a 5 year transition period from the date of the implementation of CSAR, during the transition period these products can continue to be produced, imported and sold, after the transition period it shall not be produced, imported and sold yet. Enterprises should also make timely preparations for the transition.
1.2 Non-special use cosmetics
In 2020, the locations of domestic responsible persons for the filing of imported non-special use cosmetics involve 25 provincial administrative regions, with a total of more than 21000 products filed. There are 11143 SKU filed in Shanghai as the first delegated pilot area in 2020, accounting for about 51.5% of the national total. The annual filed amount in Guangdong Province was 3375 in 2020, accounting for 15.5% of the national total. Zhejiang province and Beijing accounted for 10.6% and 9.9% of the national total respectively. The details are shown in Figure 2.
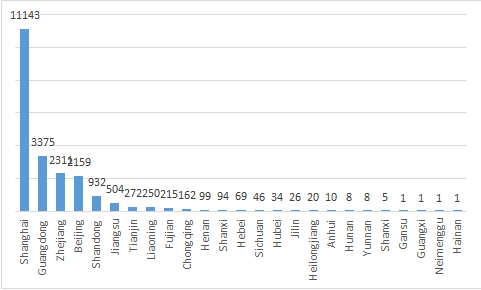
Figure 2 Statistics of the amount of filing certificates of imported non-special use cosmetics in all provincial administrative regions
In Gansu, Hainan, Guangxi Zhuang Autonomous Region and Inner Mongolia Autonomous Region, only 1 product was filed. Anhui, Hunan, Yunnan and Shanxi provinces also had no more than 10 SKU filed.
In addition, there are more than 580,000 domestic non-special use cosmetics filed in 2020, reaching a new level.
2. Policy events
The introduction of a series of new regulations in 2020 is bound to have a profound impact on the entire cosmetics and toothpaste industry. So what major policies have been introduced? What events are worthy of corporate attention? CIRS China personal and home care team here did a summary in detail.
2.1 Cosmetics
Since the launch of Cosmetics Supervision and Administration Regulations (National Decree No. 727), the draft supporting regulations were released including the management system of cosmetics and new cosmetic ingredients classification, the scope of responsibility and qualification of applicant and domestic responsible person for cosmetic and new cosmetic ingredients registration and filing, pre-market registration/filing requirements and post market surveillance mode of cosmetics and new cosmetic ingredients, cosmetics safety assessment, efficacy evaluation and label requirements, etc. The details are as follows:
On June 29, 2020, Cosmetics Supervision and Administration Regulations;
On July 21, 2020, Management Measures for the Cosmetics Registration (draft) , Management Measures for the Supervision of Cosmetic Production and Business Operation (draft);
On July 29, 2020, Technical Guideline for the Cosmetics Safety Assessment (draft) , Cosmetics Classification Rules and Catalog (draft);
On August 28, 2020, Specification for the Cosmetics Registration and Filing Data (draft) , Specification for the New Cosmetic Ingredient Registration and Filing Data (draft);
On September 1, 2020, Guidelines for the Evaluation of Cosmetics Efficacy Claims (draft);
On September 21, 2020, Management Measures for the Cosmetics Labeling (draft);
On September 28, 2020, Standard for Quality Control of Cosmetics Production (draft), Management Measures for the Monitoring of Cosmetics Adverse Reactions (draft), Management Specification for Cosmetics Sampling Inspection (draft);
On November 5, 2020, Specification for the Cosmetics Registration and Filing Data (draft) , Specification for the New Cosmetic Ingredient Registration and Filing Data (draft), Specification for the Evaluation of Cosmetics Efficacy Claims (draft);
On November 12, 2020, Management Measures for the Cosmetics Supplement Testing Methods(draft);
On December 28, 2020, Notice on the implementation of related matters of Cosmetics Supervision and Administration Regulations (Notice no. 144, 2020), The comprehensive bureau notice of NMPA regarding the selection of cosmetics sampling inspection and the re-inspection agency;
On December 31, 2020, Sate Administration for Market Regulation deliberated and passed the Management Measures for the Cosmetics Registration and Filing.
In 2020, National Institutes for Food and Drug Control (NIFDC) also issued a series of notices on cosmetics inspection. On March 9, 2020, according to the NMPA’s work requirements, NIFDC solicited revision suggestions in public for Safety and Technical Standard for Cosmetics. The scope of solicitation includes the banned and restricted ingredients, allowed cosmetic ingredients and testing methods, etc. On September 16, 2020, the revision suggestions for the inspection methods of Safety and Technical Standards for Cosmetics (2015 Edition) were solicited from the public. On November 12, 2020, a series of testing methods were issued for public opinions including the testing method of boric acid and borate in cosmetics(draft), testing method of 32 hair dye agents like para-phenylene diamine in cosmetics(draft), testing method of 15 hair dye agents like 2 - amino - 4 - hydroxy ethyl amino anisole sulfate in cosmetics (draft), testing method of retinoic acid and its derivatives in the cosmetics (draft), micronucleus test of in vitro mammalian cells (draft), efficacy evaluation method of anti-freckle and whitening for anti-freckle and whitening cosmetics (draft), and efficacy evaluation method of anti-hair loss for anti-hair loss cosmetics(draft).
2.2 Toothpaste
In addition to cosmetics, new policies were issued as well in 2020 regarding the supervision of toothpaste. The Article 77 of Cosmetics Supervision and Administration Regulations points out that toothpaste shall be administered as ordinary cosmetics in accordance with the provisions of CSAR. The toothpaste filers can claim the function of toothpaste for anti-caries, inhibiting dental plaque, resisting dentin sensitivity and alleviating gingival problems after conducting the efficacy evaluation according to the national and industrial standards. The specific management measures shall be drawn up by the pharmaceutical supervision and administration department under the State Council and submitted to the market supervision and administration department under the State Council for approval and promulgation.
On November 13, 2020, NMPA issued the Management Measures for the Supervision of Toothpaste (draft), which clarified the regulatory authority of toothpaste, defined the new raw materials of toothpaste and formulated the criteria for the determination of new raw materials of toothpaste, proposed the management requirements for the raw materials used in toothpaste, listed the required materials submitted for the filing of toothpaste, and put forward corresponding requirements for the efficacy classification and naming, efficacy evaluation and evaluation institutions of toothpaste.
On July 2, 2010, China Oral Care Industry Association (COCIA) published the instructions on the management mode of toothpaste after the promulgation of CSAR; On August 25, 2020, in order to further standardize the management of toothpaste raw materials, NMPA authorized COCIA to collect the raw materials used in the marketed toothpastes; On December 29, 2020, a statement regarding related issues on the Management Measures for the Supervision of Toothpaste was issued.
Currently, it is not easy to meet the new regulatory requirements for the Chinese cosmetics and toothpaste industry. How to balance the dilemma of survival and development of enterprises will depend on the transition period and the corresponding treatment. In the face of a series of secondary regulations that have not yet been implemented, the professional team of Personal and Home Care Division of CIRS Group China has launched a series of webinars, aiming to summarize and analyze the main contents of the regulations for enterprises. For the detailed info, please refer to CIRS Webinar Plan from Jan to Feb 2021: Cosmetics and Disinfectants.
If you have any needs or questions, please contact us at service@cirs-group.com.

