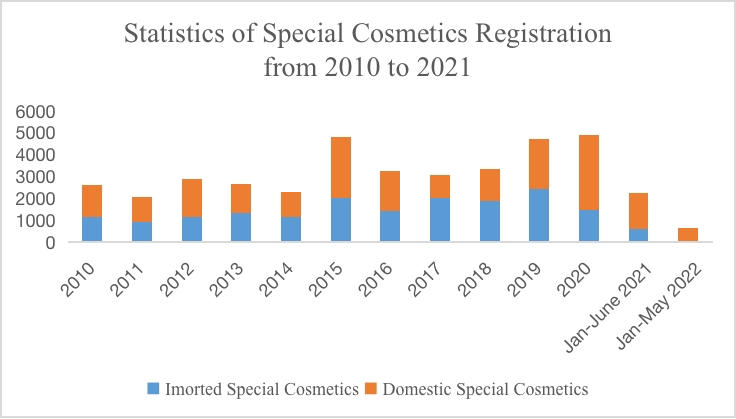
According to the statistics of National Medical Products Administration (NMPA), as of May 26, a total of 675 cosmetics registration certificates have been issued in 2022. Among them, certificates for imported special use cosmetics only accounted for 11 percent of the total. Due to the outbreak of the Covid-19 epidemic in 2020, the total amount of registrations for special use cosmetics in the first half of 2021 merely remained half of that in the previous year. Worse still, as the epidemic continued in the first half of 2022 and due to the transition of cosmetic regulations, the number of registrations for special use cosmetics decreased significantly compared with that in the first half of 2021, by 76.5%. At the same time, during the transitional period, the technical review period for special use cosmetics is prolonged and the passing rate is decreased, which may also lead to the decrease of the volume of registrations for special use cosmetics. The specific statistics are as follows:
Registration of New Products
- From January 1, 2022, when applying for registration or filing, registrants and filers shall, in accordance with the requirements of the Provisions, provide safety-related information of ingredients with functions of anti-corrosion, sunscreen, coloring, hair dying, anti-freckle and whitening.
- From January 1, 2022, when applying for the registration of cosmetic products with functions of anti-freckle, whitening and anti-hair loss, applicants should submit the required human efficacy test reports.
- From January 1, 2022, cosmetics registrants and filers must carry out cosmetic safety assessment and submit product safety assessment materials in accordance with the requirements of the Technical Guidelines before applying for registration of special use cosmetics or filing of ordinary cosmetics.
- From January 1, 2022, cosmetics registrants and filers who apply for registration of special use cosmetics or filing of ordinary cosmetics should evaluate the efficacy claims of cosmetics in accordance with the requirements of the Standards, and upload the abstract of the basis for efficacy claims on a specific website designated by the NMPA.
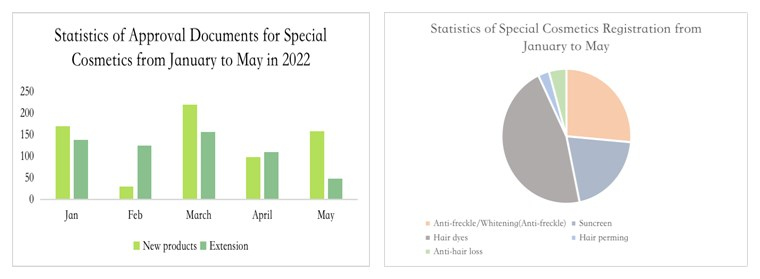
Since 2022, the registration of special use cosmetics has been greatly affected by the above rules. The registrations approved in January are mainly those applied under the old registration system (the registration certificate number is G and J), before May 1, 2021. In addition, only one special use cosmetics has been approved under the new cosmetic registration system in the second half of 2021. Although the new systems went online on 1 May 2022, the applications for registration of special use cosmetics under the new system gradually got approved from February 2022.
According to the Measures for the Administration of Cosmetic Registration and Filing, after the acceptance of special use cosmetics registrations, product technical evaluation institutions shall organize and carry out a technical evaluation within 90 working days upon receipt of the application materials. The number of registrations for special use cosmetics is reduced, because when applying for registration of products, the data may only meet the current regulatory requirements yet not conform to the "dynamic" new regulations and policies. In addition, overseas enterprises may respond slower to the updates to cosmetic regulations in China than domestic enterprises. Besides, the number of registration for imported special use cosmetics is less than that of domestic special cosmetics. This phenomenon also occurred when products for whitening purposes were managed as special use cosmetics in 2014.
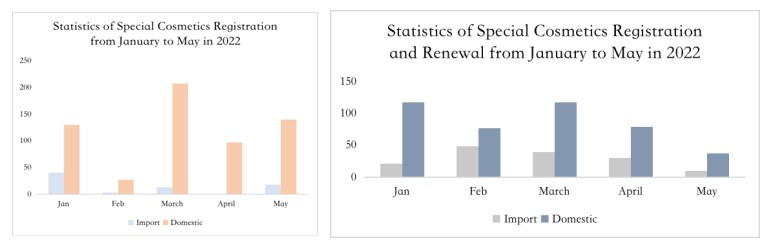
Extension of Registration
In May 2019, the National Medical Products Administration (NMPA) issued the Notice regarding the enforcement of the Renewal Commitment System for Administrative License of Special Use Cosmetics (Notice No. 45, 2019)", which officially started the enforcement of the renewal commitment system for special use cosmetics. Although the aforementioned announcement has now been repealed, Article 44 of Section 4 of Extension of Registration Certificate under the Measures for the Administration of Cosmetic Registration and Filing (released on 12 Jan. 2021) announces that the accepting institution shall, within 5 working days after receiving the application for extension of registration, conduct formal examination of the application materials, accept the application if it meets the requirements, and issue a new registration certificate to the applicant within 10 working days from the date of acceptance. The regulation is basically the same as the requirement of the renewal commitment system released in 2019. As can be seen from the statistics, by the end of May, the number of special use cosmetics applying for extension of registration is basically the same as that of new registrations.
Opinions of Recent Technical Review
- Clearly control the use of sunscreen agents used in the products according to the formula under the Product Execution Standard “Microbial Indicators and Physicochemical Indicators”
- Please explain how to ensure the quality and safety of the product in the later production after the first inspection of the product.
- The quality management measures for dioxane project under the standard "Microbial and Physical and Chemical Indicators" are not rigorous . Please explain how to ensure the quality and safety of the product in the later production after the first inspection of the product.
- If the anti-freckle /anti-hair loss active ingredients in the product are plant extracts, the quality specifications of the anti-freckle /anti-hair loss active ingredients including characteristic ingredient control indicators issued by the raw material supplier shall be provided. In addition, the test report on anti-freckle/whitening /anti-hair loss efficacy is also required.
- It is not appropriate to claim "low irritation", "completed skin irritation test", "skin health" and "passed skin test" in the product label, and it is suggested to delete.
If you have any needs or questions, please contact us at service@cirs-group.com.

