Since May 1, 2021, National Medical Products Administration requests cosmetic registrants, filers, and domestic responsible persons to apply for the registration of special cosmetics and filing of ordinary cosmetics through the new registration and filing platform. The operating mode of the new cosmetics registration and filing platform has great changes compared with the old cosmetics registration and filing system. Due to the different supervision methods and operation systems of ordinary cosmetics and special cosmetics, it gives more challenges for companies to adapt to the registration and filing process of cosmetics
1. What is the operation process of online system for the filing of ordinary cosmetics and the registration of special cosmetics?
Before filing of ordinary cosmetics, the filing number can be obtained in advance, and then the filing application data shall be filled in the cosmetics filing system. The filing will be completed after submission. If the submitted data meet the requirements of the filing data, the filing information shall be made public; if not, the relevant regulatory authorities may require the order correction. At this moment, for products with public filing information: paper materials need to be submitted for the filing of imported ordinary cosmetics. Nevertheless, the paper materials for the filing of domestic ordinary cosmetics can be kept by the domestic filer for official review.
For the registration of special cosmetics, the first step is to fill in the application form and related materials on the system for submission, then the paper materials shall be submitted to the administrative affairs acceptance service agency of the National Medical Products Administration. According to the relevant content in the "Technical Guidelines for the Submission of Cosmetics Registration and Filing Documents (Trial)", the accepting agency will conduct formal review work in accordance with relevant regulations after receiving the electronic and paper versions of the registration documents. However, in the actual operation process, after the submission of electronic registration information, the accepting agency will conduct a formal review. Once passing the format review, the enterprise shall submit the paper materials.
When submitting the paper materials, a declaration of conformity shall be submitted at the same time to clarity the consistency of both the electronic and paper materials.
The specific operation process is as follows:
1) Operation process of ordinary cosmetics filing system:
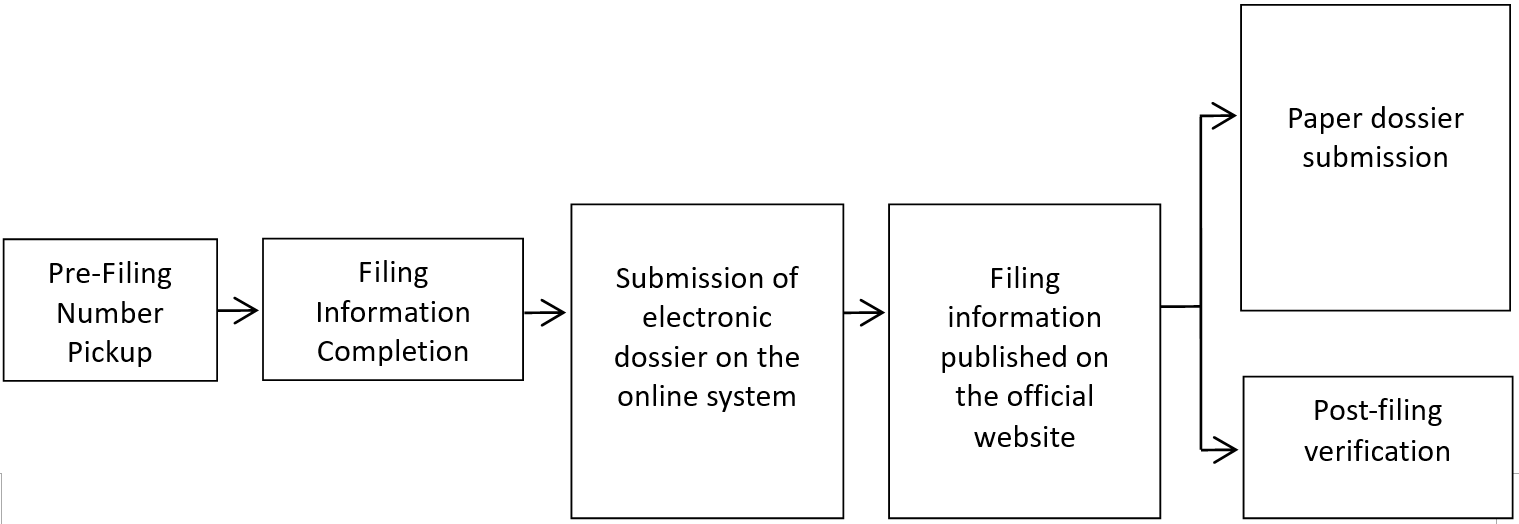
2) Operation process of special cosmetics registration system:
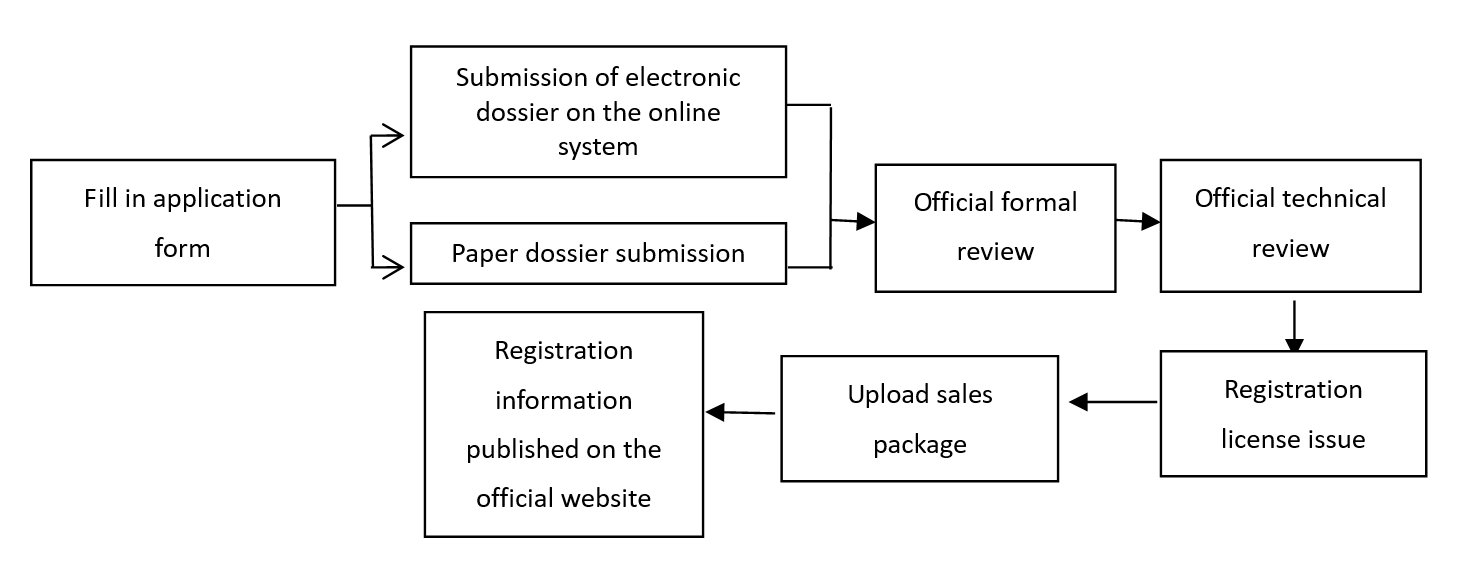
2. What is the difference of dossier requirements between the filing of ordinary cosmetics and the registration of special cosmetics?
The application data of ordinary cosmetics and special cosmetics on the cosmetics registration and filing information service platform are basically the same. According to the "Provisions on the Administration of Cosmetics Registration and Filing Materials", the system needs to submit 7 parts of materials: "Cosmetics Registration and Filing Application Form" and related materials, naming basis of product name, formulas, implementation standards, labels, inspection reports, product safety assessment report.
For the filing of ordinary cosmetics and the registration of special cosmetics, there are two different ports during system operation. There are many practical differences in the submission of materials so far mainly including:
1) The production process in the implementation standard
• The ingredient grouping of ordinary cosmetics requires the company to fill in the ingredient grouping in text form.
• The ingredient grouping of special cosmetics can be directly selected and grouped in the system..
2) Inspection report
• The inspection report of ordinary cosmetics should be uploaded by the enterprise itself.
• The inspection report of special cosmetics has been linked to the cosmetics registration and filing inspection system so far, and the company only needs to upload inspection application materials such as inspection acceptance notice and application form.
3) Requirements for seal/signature of registration and filing document
• When filing ordinary cosmetics, in addition to the documents required to be uploaded in the system, such as sales package and safety evaluation reports, other materials shall be filled in the system, only the application form needs to be generated separately and sealed by the filing person for confirmation. For imported ordinary cosmetics, the application form also needs to be sealed and confirmed by the domestic responsible person. However, due to the different requirements of various local MPAs at this moment, some local MPAs require all dossiers to be stamped and confirmed by the filer.
• When registering special cosmetics, all dossiers should be filled in the system and be saved before uploading the attachment. The full dossiers shall be sealed and confirmed by the registrant or domestic responsible person. Please note that the application form and the implementation standard file need to be confirmed by the overseas registrant for the registration of imported special cosmetics. Recently, the special cosmetics registration system has been upgraded and optimized: the original one-step submission is subdivided into two-step submissions.
Step 1: The applicant first fills in the registration information in the system, saves the filled information, and an [Upload Attachment] button appears in the application management list.
Step 2: On the [Upload Attachments] of the application form management page, after downloading the attachments generated by the system, seal them and send them back or sign.
Finally, click the [Submit] button on this page to complete the registration application. In addition, if the application information needs to be modified after saving the filled information, the application number will be regenerated, and all materials need to be downloaded, sealed and uploaded again.
3. What is the difference between the product packaging information submitted during the registration of ordinary cosmetics and special cosmetics?
The plan of the product sales package, the three-dimensional picture of the sales package, and the product manual need to be uploaded for the filing of ordinary cosmetics. For imported products, the plan of the original sales package plan and the translation of the original sales package are needed as well. Nevertheless, the Chinese labels is no need to be uploaded for the products with Chinese labels.
For the registration of special cosmetics, only the original sales package of the product and its manual, and the corresponding translation are required for official review. After obtaining the registration certificate, the plan and three-dimensional diagrams of the sales packaging of the product should be uploaded to the system.

