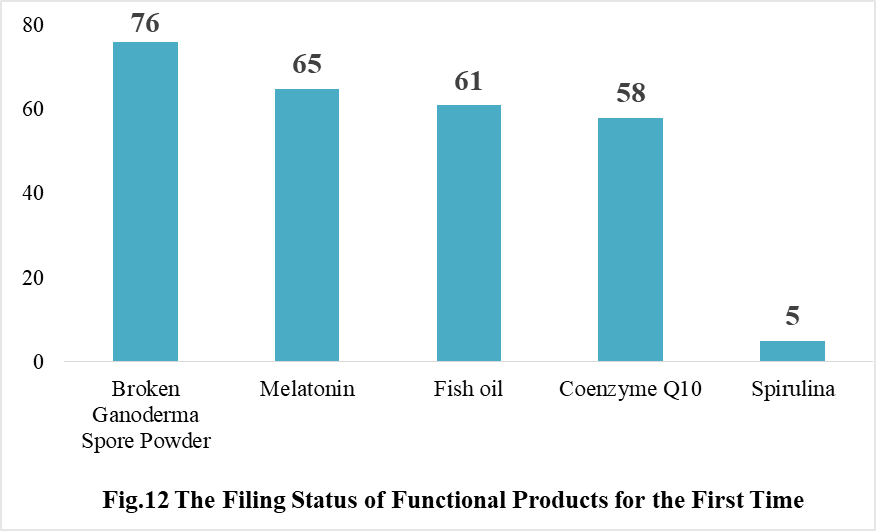Updated on 14 Feb. 2022
In order to help enterprises better understand the filing status of health food (dietary supplement) in China, CIRS counted the data of filed products published in 2021 and made an analysis for your reference.
1. The Filing Status of Health Food in China
According to the information released by the Center for Food Evaluation of SAMR and the Local Administration for Market Regulation, a total of 2382 health food obtained the filing certificates, of which 2359 are domestic health foods and 23 are imported health foods by December 31, 2021.
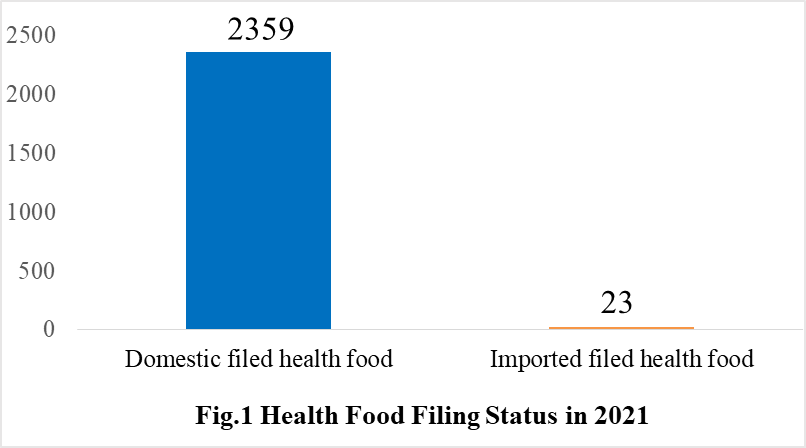
2. The Filing Status of Health Food in Different Regions
The number of approved domestic filing health food are varied in different provinces in China. Shandong and Guangdong rank the first and second place respectively with the number of 806 and 439. The applicants who come from United States, Canada, Japan, Australia, Denmark, South Korea, and New Zealand obtained the filing certificates of imported health food. There are 23 imported filing products, the applicants of 12 products are Canadian enterprises, and the applicants of 8 products are Japan enterprises.
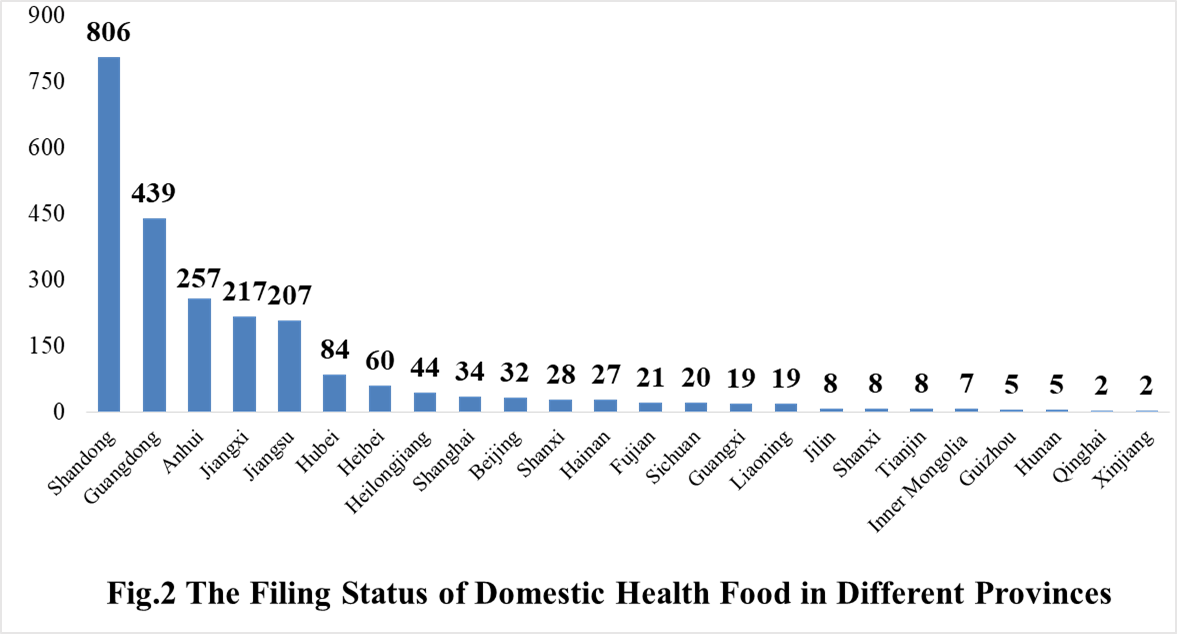
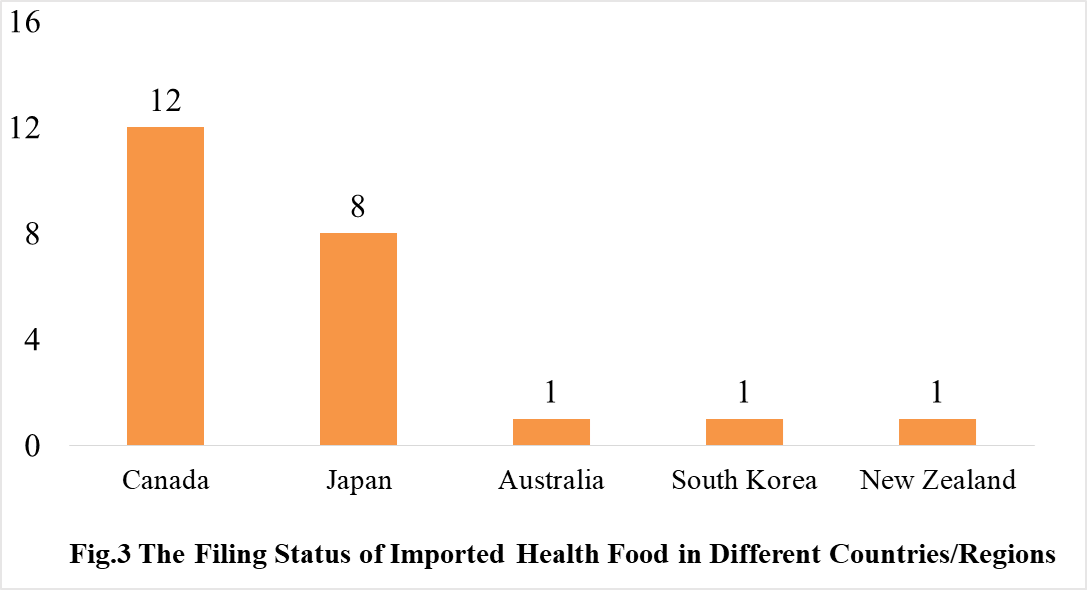
3. The Filing Status of Health Food in Different Enterprises
3.1 Domestic Filing Products
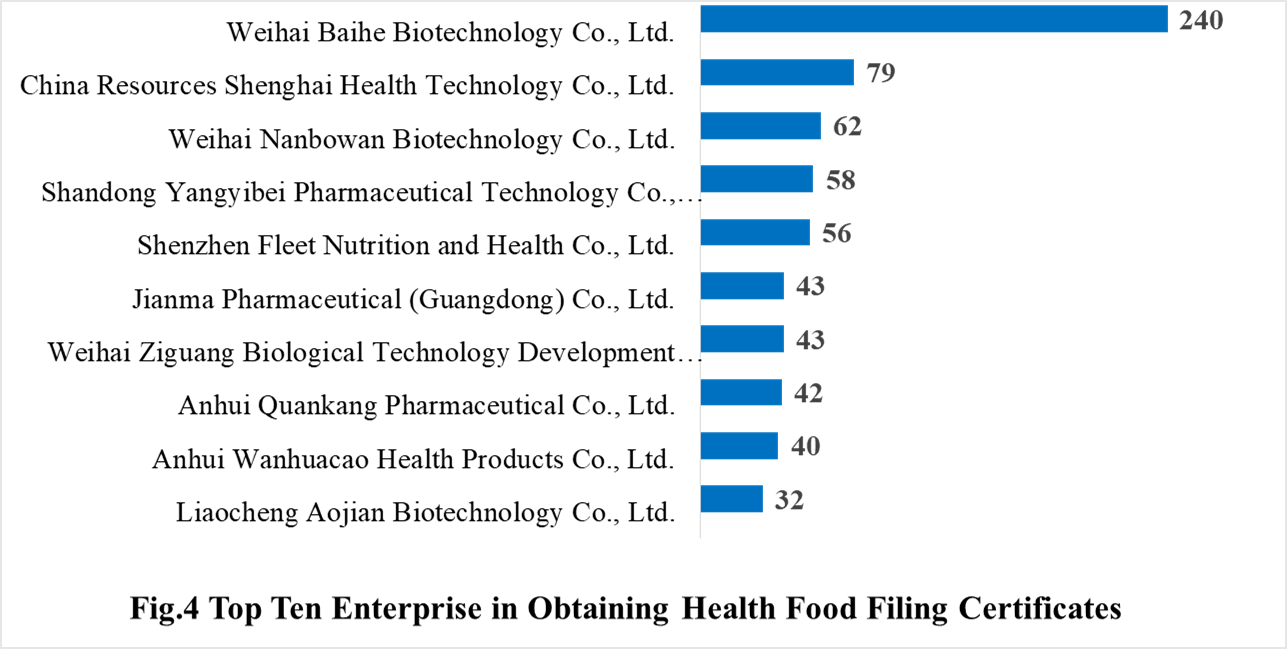
In 2021, 384 domestic health food manufacturers obtained health food filing certificates. Weihai Baihe Biotechnology Co., Ltd. has the largest number of filings, and a total of 240 products obtained the filing certificates, followed by China Resources Shenghai Health Technology Co., Ltd. and Weihai Nanbowan Biotechnology Co., Ltd. with the number of 79 and 62, respectively.
3.2 Imported Filing Products
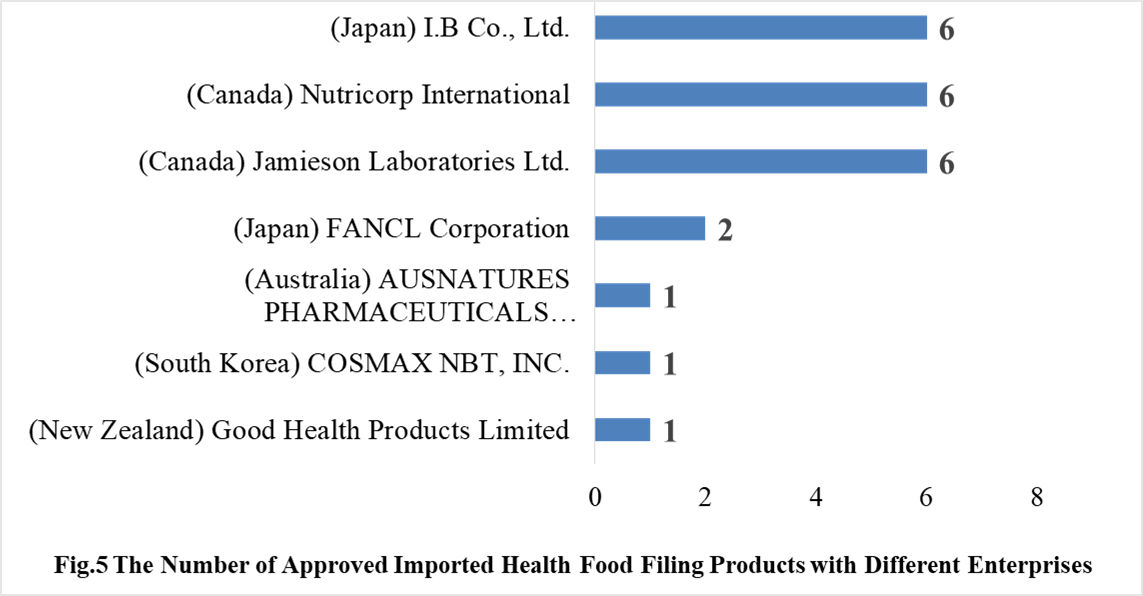
In 2021, 7 oversea companies got health food filing certificates. Jamieson Laboratories Ltd., I.B Co., Ltd. and Nutricorp International have the largest number of filings, and a total of 6 products obtained the filing certificates.
4. The Filing Status of Health Food in Different Dosage Forms
At present, the permitted dosage forms of Chinese health food products include tablet, capsule (hard/soft), oral liquid, granule, powder and gelatin candy.
4.1 Filing status of domestic products
In the 2021, the main dosage form for domestic products is tablet, with the number of 1259, accounting for 53.37% of the total.
The capsule include soft capsule and hard capsule, and the number of filings is 752. The quantity of soft capsule products is far higher than the number of hard capsule products, which are 638 and 114 products respectively.
In addition, the quantity of oral liquid (including drops) and granule products are 154 and 132 respectively. Among them, there are only 49 drops products.
Powder and gelatin candy are the new dosage forms available for filing since July 1, 2021, and 62 products in these 2 dosage forms have got the filing certificates.
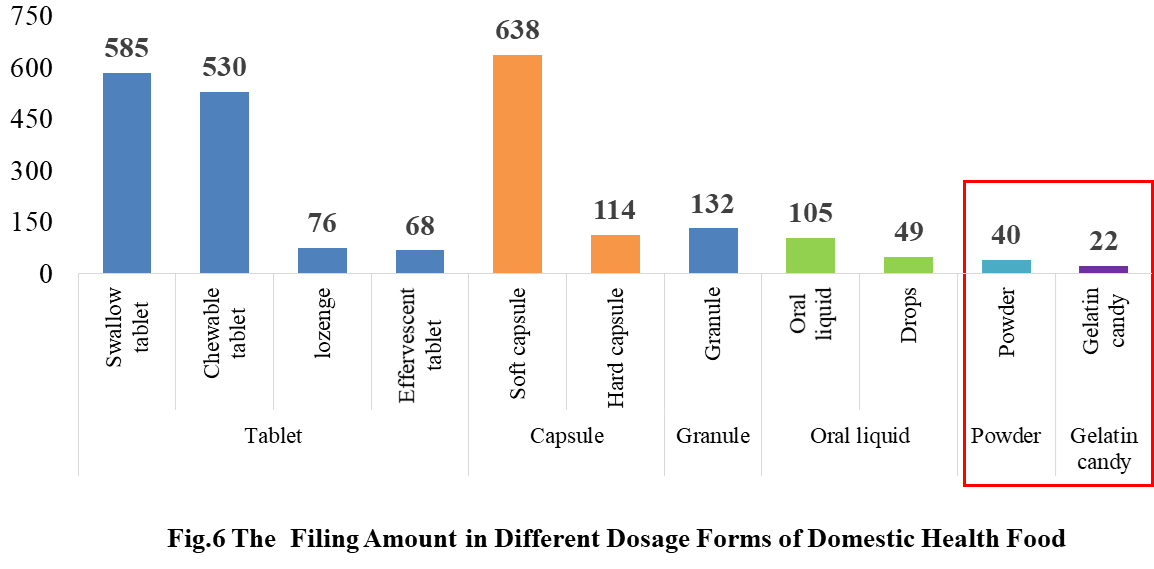
4.2 Filing status of imported products
In 2021, the dosage form of approved imported health foods is mainly tablet with the number of 20. The number of capsule product is 3. No oral liquid (including drops), powder, gelatin candy or granular products were approved in 2021.
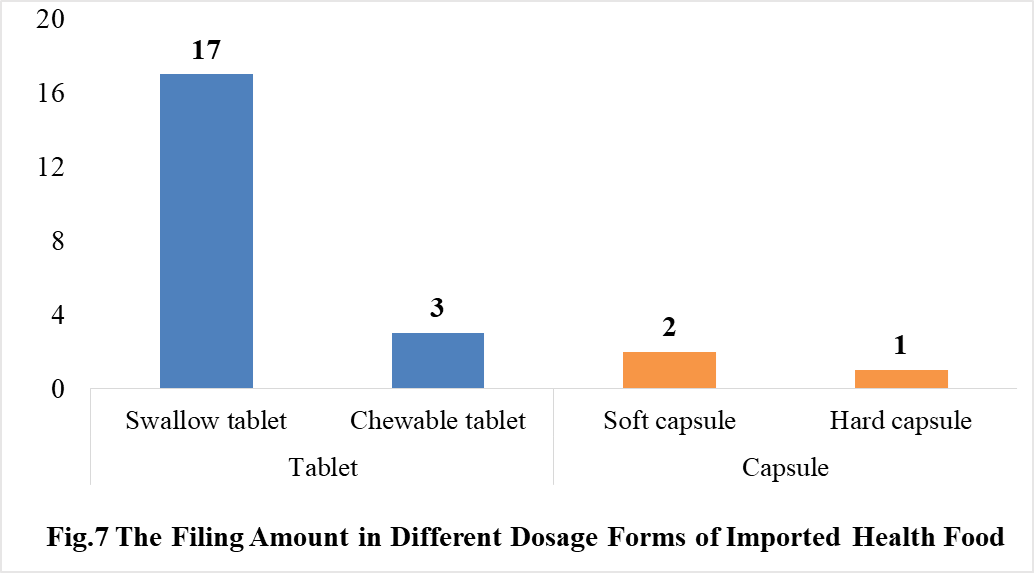
5. The Number of Approved Filed Health Food with Different Nutrients
5.1 Domestic Filing Products
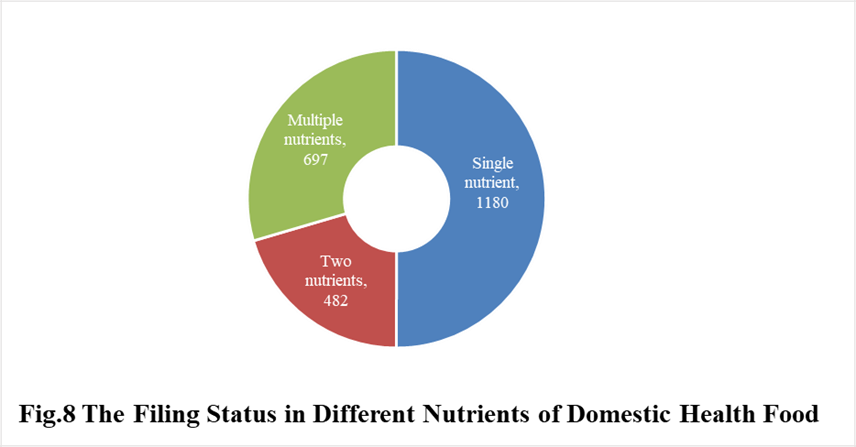
In 2021, the number of domestic products supplementing single nutrient is the most, which is 1180, followed by the products supplementing multiple nutrients and two nutrients, the number are 697 and 482 respectively.
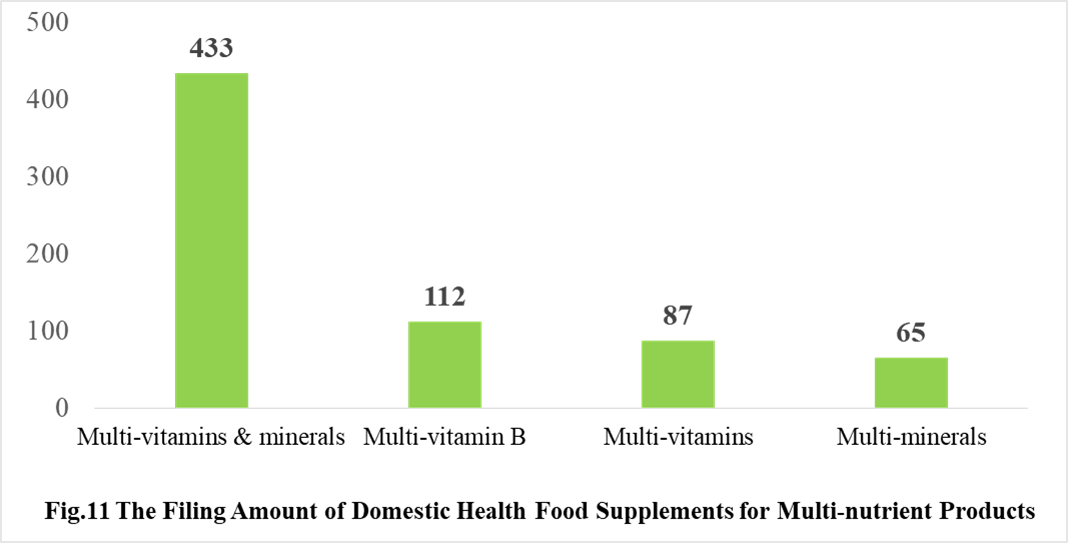
Among the domestically filed products in 2021, the most popular nutrition supplements are Vitamin C supplements, Calcium and Vitamin D supplements, Multi-vitamins & minerals supplements, which is 388, 188 and 433 respectively.
Ganoderma lucidum spore powder, melatonin, fish oil, coenzyme Q10 and spirulina products were transferred from registration to filing supervision for the first time in 2021, and the filing number is 76, 65, 61, 58 and 5 respectively.
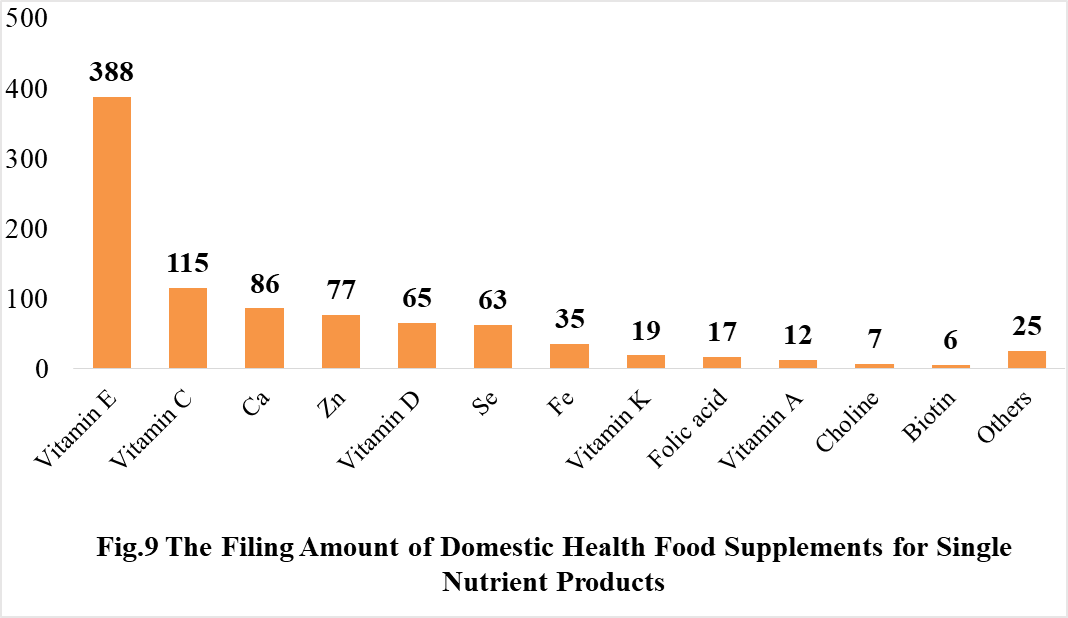
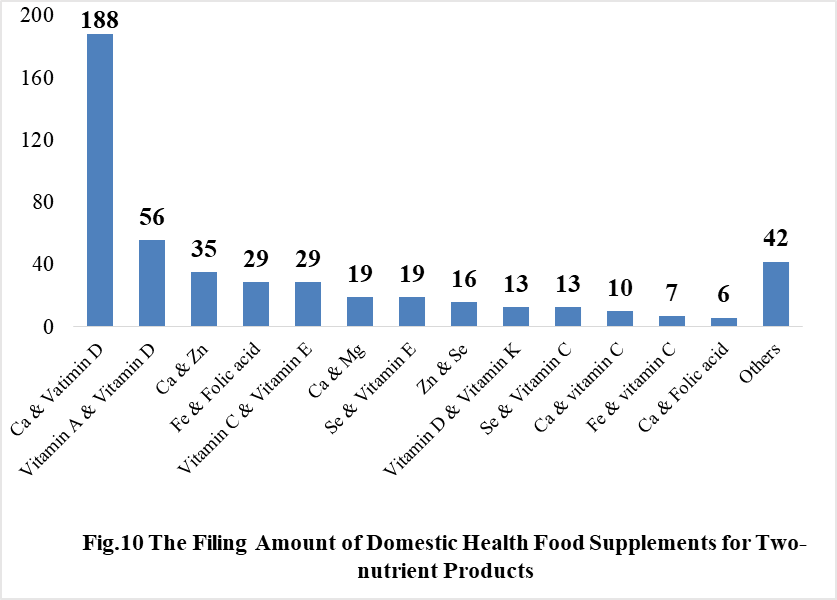
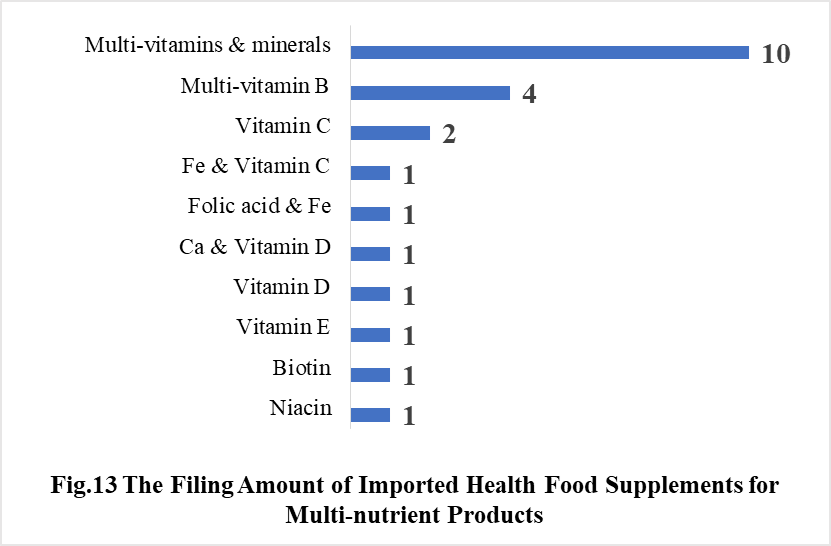
5.2 Imported Filing Products
In 2021, the number of imported products supplementing Multi-vitamins & minerals is the most, which is 10, followed by the products supplementing Multi-vitamin B and Vitamin C, which is 4 and 2 respectively.
CIRS Comments
Starting from June 1, 2021, domestic health foods that use Coenzyme Q10, Broken Ganoderma lucidum spore powder, spirulina, fish oil or melatonin as single raw materials can apply for filing in China, and 265 products have got the filing certificates within half a year (only domestic products with these ingredients can apply for filing). At the same time, gelatin candy and powder have become the approved dosage forms for fiing products. The number of filing products with these two dosage forms has grown rapidly in 2021. Companies have gained more space in the selection of raw materials, function claims and dosage forms for filing products due to the implementation of these regulations.
On 20 December 2021, SAMR issued three drafts, including Health Food Raw Materials Directory of Nutrition Supplement (2022 version) (Draft), etc. It means that 6 raw materials including DHA, soy protein isolate, and whey protein will be added to the list of health food raw materials. In addition,“DHA” is added into the “Directory of Nutrition Supplement”, as the function of this nutrient ("supply n-3 polyunsaturated fatty acids") is within the scope of “supply nutrients”, CIRS expects that overseas enterprises can also apply for the filing of DHA products in the future. Compared with current regulations, the scope of health food filing is further expanded in the drafts, which will provide more opportunities to health food companies.
Note:
I. The data in this article is from the Special Food Information Query Platform, Center for Food Evaluation of SAMR, and Local Administration for Market Regulation.
II. There may be some omissions in the data of domestic filed health food due to the replacement of new and old websites of government departments after the reform of state institutions, thus the data in this article is for reference only, and please refer to the information published by the government.
III. The Special Food Information Query Platform and Center for Food Evaluation of SAMR lag behind in information release. According to CIRS’s knowledge, the information of some products that have obtained health food filing certificates have not been published on the official website of the government departments. Therefore, the actual record amount of health food exceeds the data listed in this article.