On June 29, 2020, the State Council issued the "Cosmetics Supervision and Administration Regulations"(CSAR) (National Order No. 727), which will take effect on January 1, 2021.
The History of the Implementation of Cosmetic Supervision and Administration Regulations
Regulations on Hygienic Supervision of Cosmetics were issued in 1989 and implemented in 1990;
Regulations on Hygienic Supervision of Cosmetics (Revision) as the legislative work plan items of the State Council in 2013, 2014 and 2015;
On November 8, 2014, the "Cosmetics Supervision and Administration Regulations (Draft for Comment)" (hereinafter referred to as the Regulations) issued by the State Food and Drug Administration (SFDA) publicly solicited opinions. The "Regulations" not only include the regulations on the management of cosmetic raw materials, production and operation, and advertising, but also increase the supervision content for the emerging online sales model;
On July 20, 2015, the Cosmetics Supervision and Administration Regulations (Revision Draft for Review)" (hereinafter referred to as the Draft for Review) was published to solicit public opinions and submitted to the State Council. The manuscript submitted for review focused more on the in-and post-market supervision, and the idea of Streamline Administration and Institute Decentralization is evident;
Department of Education, Culture, Health, and Legal Affairs of the Ministry of Justice, Department of Legal Affairs of the National Medical Products Administration, Department of Pharmaceutical Regulatory Affairs of the National Medical Products Administration established an investigation team regarding the legislative investigation of the Cosmetics Supervision and Administration Regulation for cosmetics manufacturers, business enterprises and industry associations;
On December 18, 2018, China submitted the notification of G / TBT / N / CHN / 1310 to the WTO / TBT National Notification Advisory Center; and
On January 3, 2020, Premier Li Keqiang of the State Council chaired the 77th Executive Meeting of the State Council and passed the Cosmetics Supervision and Administration Regulations (Draft).
There are six chapters and eighty articles in this Ordinance. It is divided into general rules (10 articles), raw materials and products (15 articles), production and operation (20 articles), supervision and management (13 articles), legal responsibilities (18 articles), and supplementary articles (4 articles). CIRS Group analyzes the main contents of CSAR, compared with the existing regulatory system and the draft version of the CSAR issued in the past.
Main Contents of Cosmetic Supervision and Administration Regulations
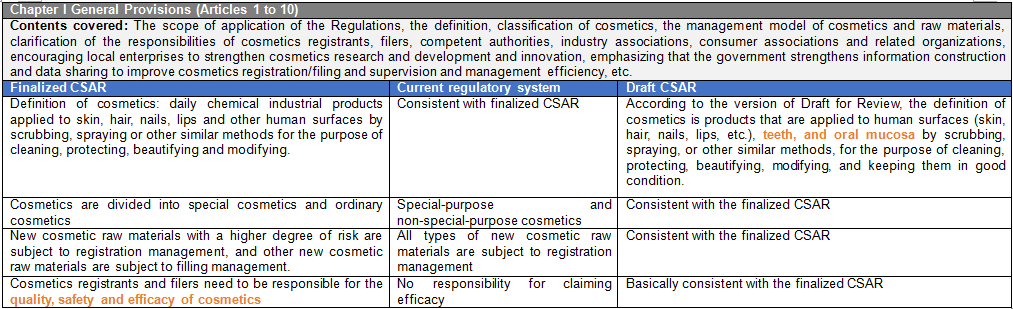
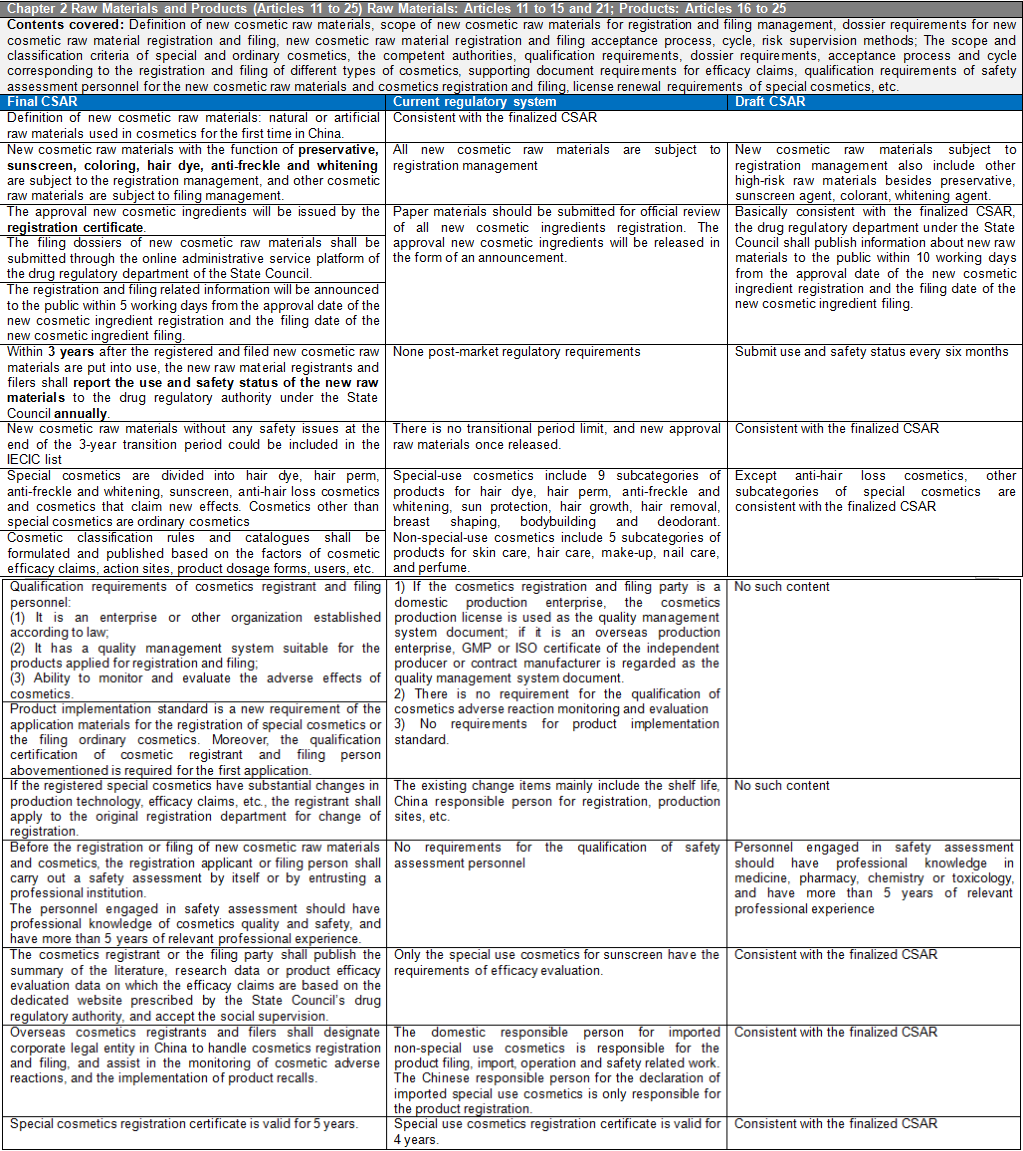


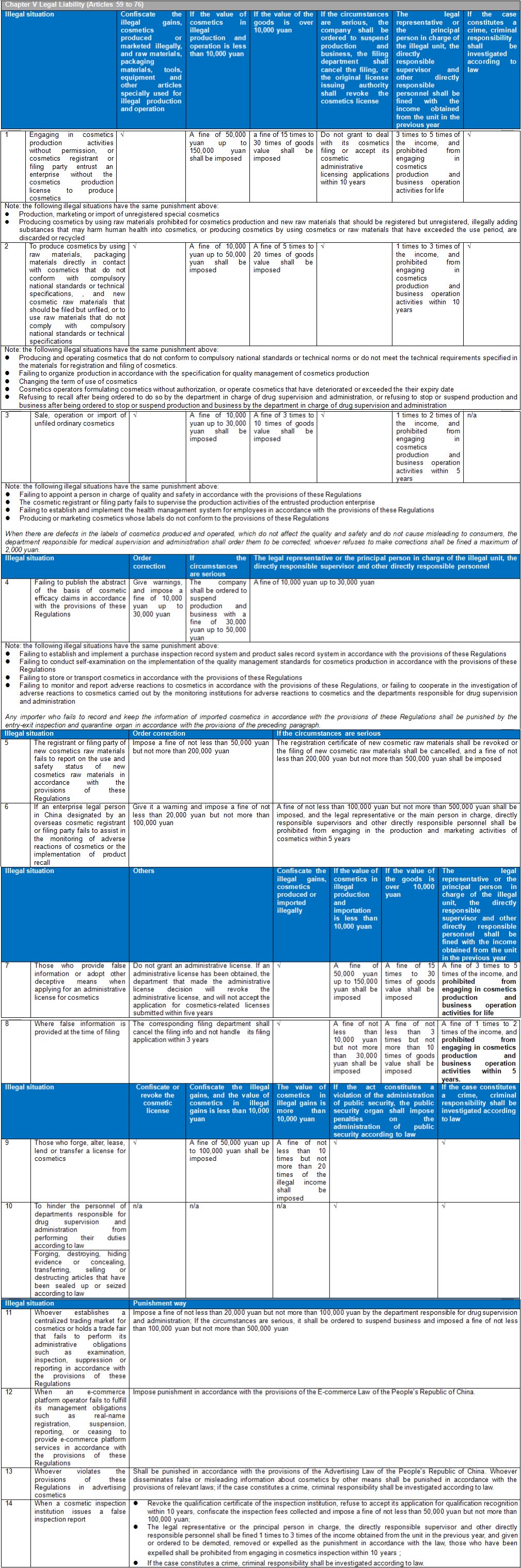

- Cosmetics Supervision and Administration Regulations will replace the Regulations Concerning the Hygiene Supervision of Cosmetics which have been in force for three decades. The Regulations will be the strictest in the history of Cosmetics in China. The implementation of these Regulations will represent the arrival of the cosmetic 2.0 era in China. The half-year transition period requires rapid upgrading and adjustment to meet the requirements of the new regulations and a series of subsequent secondary regulations. The main contents of these Regulations are summarized as follows:
It is emphasized that the cosmetics registrants and filers are responsible for the cosmetics quality safety and efficacy claim. They shall fulfill the relevant obligations of pre-market registration and filing management, conduct pre-market safety assessment of products by themselves or entrust professional institutions, perform the related obligations of post-marketing adverse reaction monitoring, evaluation and reporting, product risk control and recall, product and raw material safety reassessment, and assume the principal responsibility for the quality and safety of registered products. Foreign cosmetic registrants and filers shall designate legal entities in China to register and file cosmetics, assist in the monitoring of adverse reactions to cosmetics and the implementation of product recalls. Where the production of cosmetics is entrusted, the cosmetics registrants and filers shall entrust the enterprises that have obtained the corresponding production licenses of cosmetics and supervise the production activities of the entrusted production enterprises. The contract manufacturer shall be responsible for the production activities and accept the supervision of the cosmetics registrants and filers. In addition to the registrants and filers, it also points out that the importer's job duties in the supply chain link should verify whether the cosmetics to be imported have been registered or filed and whether they conform to these Regulations and mandatory national standards and technical norms. Those who failed to pass the examination and verification shall not be imported. Importers shall faithfully record the information of imported cosmetics, and the record-keeping period shall conform to the provisions of Article 31, Paragraph 1, of these Regulations. Cosmetics registrants, filers and commissioned production enterprises shall set up quality and safety control persons with professional knowledge of cosmetics quality and safety and at least 5 years of experience in cosmetics production or quality and safety management. If there is no quality and safety control person, the illegal unit will be fined up to 10 times the value of the goods and the responsible person of the illegal entity will be banned from the production and operation of cosmetics within 5 years.
Under current policy, the scope of responsibility of the domestic responsible person for imported non-special use cosmetics includes product registration, import, operation and safety. The responsible agent for the declaration of special use cosmetics is only responsible for the product registration. These Regulations specify the responsibilities of cosmetic registrants, filers and domestic legal entities designated by overseas cosmetic registrants and filers.
New cosmetics ingredients are classified according to the degree of risk. New ingredients with the function of anti-corrosion, sunscreen, coloring, hair dye, anti-freckle, and whitening shall be managed by registration, while other new raw materials shall be managed by filing. The post-market regulatory requirements for new cosmetic ingredients are included like reporting the use and safety status of new raw materials annually. In case of failure to submit a report in accordance with the Regulations will result in a maximum fine of 500,000 yuan. New registered or filed ingredients during the 3-year supervision period will still be managed as new raw materials, and the new raw materials without safety issues after 3 years will be included in the Inventory of Existing Cosmetic Ingredients in China (IECIC). With the development of science, if the safety of cosmetic raw materials changes, the registration or filing of raw materials with safety risks after re-evaluation will be revoked and they will be included in the list of prohibited raw materials.
The range of special cosmetics is narrowed down to hair dye, hair perm, anti-freckle and whitening, sunscreen, hair loss prevention cosmetics and cosmetics claiming new efficacy. The determination of cosmetics with new efficacy claims is mainly based on the classification rules and catalogue of cosmetics.
The qualification requirements for cosmetic registrants and filers are added to these Regulations. It is required for the first application of the registration of special cosmetics or the filing of ordinary cosmetics to provide the qualification documents of cosmetic registrant or filers. The qualification documents shall meet the requirements in three aspects: 1) Enterprises or other organizations established according to law; 2) It has a quality management system suitable for the products applied for registration and filing; 3) Ability to monitor and evaluate adverse reactions of cosmetics.
Compared with the previous draft Regulations, these Regulations impose the harshest penalties in the history of Chinese cosmetics on corporate violations and those responsible persons of illegal units. It not only increases the fine amount of illegal enterprises and the relevant responsible persons, but also the responsible persons may face the punishment result of a lifetime ban from engaging in the production and operation of cosmetics. These violations include:
Production of cosmetics without a qualified production license
Production, marketing or import of unregistered special cosmetics
Addition of banned ingredients in cosmetic production
Addition of new raw materials in cosmetics production
Addition of substances that may endanger human health or use of cosmetics or cosmetic raw materials that have expired, been discarded or recycled in cosmetics production
Providing false information or adopting other deceptive means when applying for an administrative license for cosmetics
Although toothpaste is not included in the definition of cosmetics, pre-market filing will be needed. According to the current policy of management of imported cosmetics, animal testing might be required for the import of toothpaste. Nevertheless, the detailed and separate requirements shall be issued by the competent authority.
Ordinary beauty soap can be imported without pre-market filing. This will greatly accelerate the entry cycle of such products. But the soaps with the function of special cosmetics have to be registered before production and import.
Cosmetics used for hair growth, hair removal, breast shaping, fitness and deodorization will not be regulated as cosmetics after 2026. The regulatory requirements shall be issued in the prospective new regulatory documents.
If you have any needs or questions, please contact us at service@cirs-group.com.
Source:
http://www.gov.cn/zhengce/content/2020-06/29/content_5522593.htm

