Background
On January 1st, 2022, the China National Medical Products Administration (NMPA) launched the cosmetic ingredients safety information platform. Since then, cosmetic ingredient suppliers should create an account on the platform and submit the safety information of their cosmetic ingredients to generate a code. This code can be shared with downstream cosmetic registrants, filers, or the domestic responsible person to affiliate the safety information of cosmetic ingredients and report to the NMPA.
- Implementation date: Jan 1st, 2022
- Regulation target:
- Manufacturers / distributors / traders / etc. of cosmetic ingredients
- Responsible for the safety of the cosmetic ingredients
How to Ensure Your Cosmetic Ingredients Are Compliant in China?
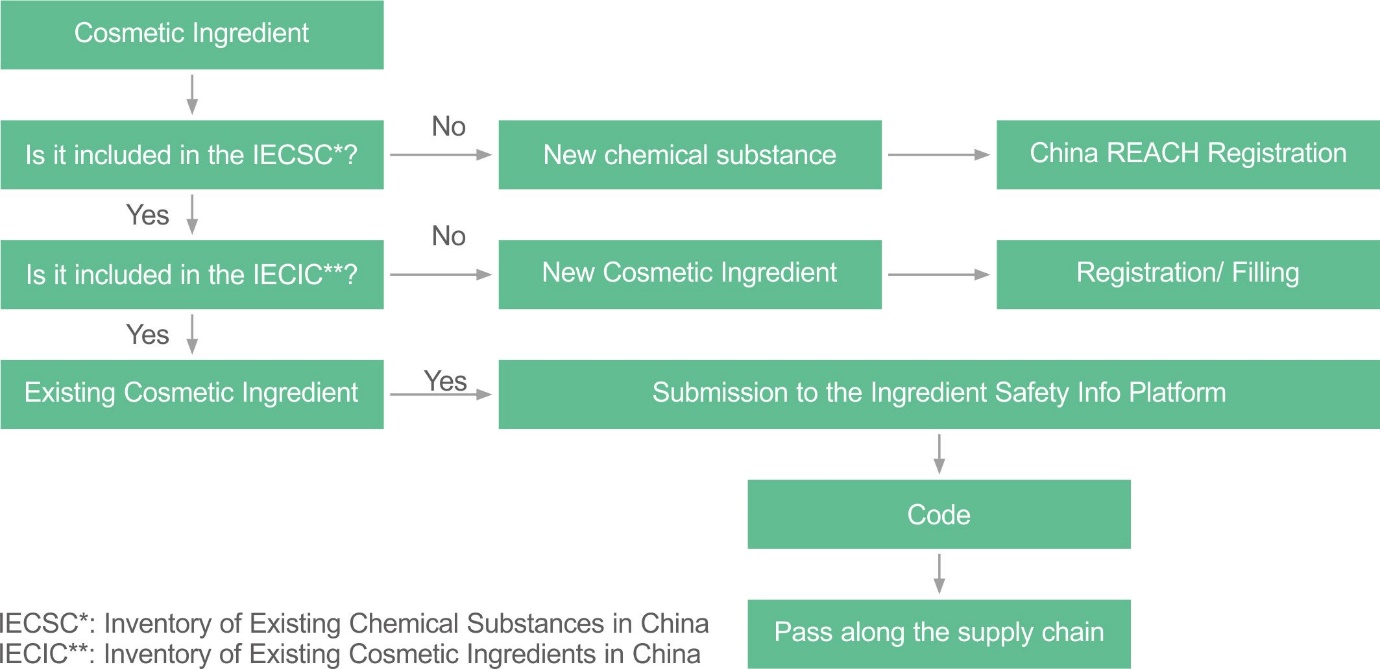
Only existing cosmetic ingredients in China, that is, ingredients listed in the IECIC 2021, can be submitted to the NMPA platform to generate the code. New cosmetic ingredients may need to consider new cosmetic ingredient filing/registration in China and/or China REACH.
Please search the Chinese Cosmetic Ingredient Regulatory Database (China CosIng) to know whether the cosmetic ingredients are listed in the IECIC 2021
What Information is Required?
Step 1: Account Set-up & Notarization
The greatest challenge facing companies based outside of China is the platform is exclusively in the Chinese language. The first step in the submission process, is setting up an account on the platform. The system requires a Chinese name for the company which can be transliterated from the original company name or chosen by the company. A copy of the original business licence of the company notarized by a Chinese Embassy or Chinese public notary and translated to the Chinese language should also be submitted to complete the account set-up process.
Step 2: Submission of Annex 14 & Code Generation
The next step is submitting the cosmetic ingredient safety information via the Annex 14 information sheet. The Annex 14 must be translated to the Chinese language before it can be submitted. Once the Annex 14 is submitted to the platform, a safety information code will be automatically generated which can be provided to downstream users for their filing/registration in China.
User Account Set-up | Submission |
Stamped Annex 13 company info sheet | Basic info & brief description of production process |
Notarized Business Licence in Chinese by China embassy or Chinese public notary | Identification & characterization index of raw materials |
Power of Attorney (if authorized company appointed) | Risk information & control indicators |
Company Name in Chinese | International authority & evaluation conclusion |
Brief description of use requirements in other industries | |
Other issues to clarify |
Practical Advice from CIRS
Latest Information Update from the NMPA:
- The safety code is generated automatically by the system after submission
- The code consists of a 5-digit manufacturer code + 6-digit ingredient code + 3-digit quality specification of raw material code
- The NMPA discloses some information of raw material composition & safety information code on the public system (http://ciip.nifdc.org.cn/hzpYL/ylgsInfo)
CIRS is monitoring the situation and having ongoing discussions with the NMPA regarding the audit process for this service, but we still recommend ensuring all information submitted to the platform is sourced very carefully and completed as much as possible to avoid any issues later on. If you already have a code and the cosmetic ingredient safety information is changed, you should start a new application on the platform and generate an updated code. If your company information is changed, CIRS can assist you in contacting the NMPA to make this update request.
CIRS will regularly post updates of this new requirement on our company website.
CIRS Services
- Technical Assistance for Submission to the NMPA Safety Information Platform
- Notarization of original business license & Translation in China
- New Cosmetic Ingredient Registration/Filing in China
- Cosmetic Registration/Filing in China
- Cosmetics Safety and Efficacy Evaluation Test in China
- China Domestic Responsible Person Service
- Global Cosmetic Regulatory Updates & Reporting
- Global Cosmetic Registration

Julie Harrington, Senior Regulatory Consultant in CIRS Europe
CIRS Europe, Regus Harcourt Centre, Dublin, Ireland
Email: service@cirs-group.com

