Since June 29, 2020, a series of cosmetics regulations have been launched, including the Cosmetics Supervision and Administration Regulations (CSAR, State Order No. 727) and a pack of supporting regulations. Among them, the Measures for the Administration of Cosmetic Registration and Filing, Provisions on the Administration of New Cosmetic Ingredients Registration and Filing Data and the Technical Guidelines for Cosmetics Safety Assessment are primarily for new cosmetic ingredients filing/ registration. According to the requirements of the above regulations, registration and filing of new cosmetic ingredients should be applied through the cosmetic declaration and assessment platform on the National Medical Products Administration (NMPA) official website from May 1 2021.
As of June 28, 2022, a total of 18 new cosmetic ingredients have been successfully filed, 13 of which are domestic new cosmetic ingredients. The rest 5 substances are imported new cosmetic ingredients. Details are as follows:
Table 1 Information of Domestic New Cosmetic Ingredients Approved for Filing under New Rules

Data Source: NMPA
Table 2 Information of the Imported New Cosmetic Ingredients Approved for Filing under New Rules
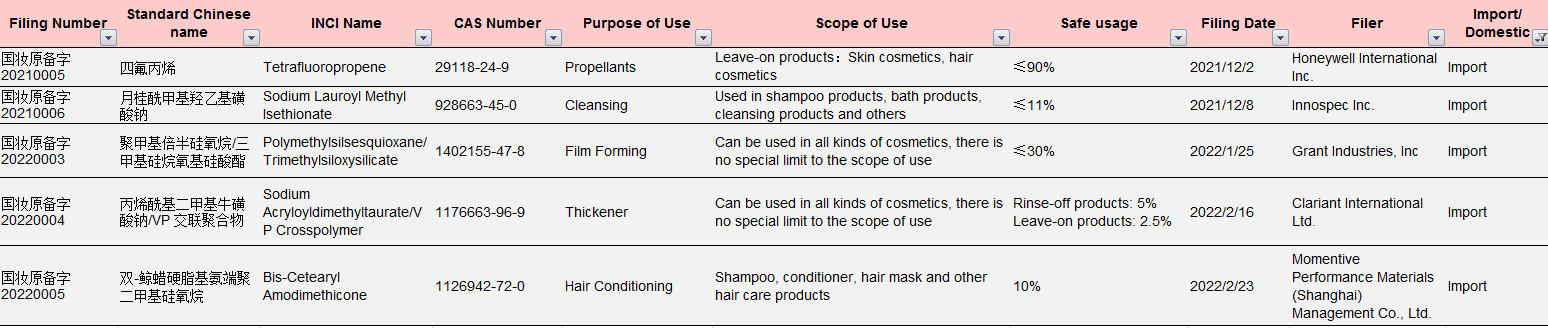
Data Source: NMPA
However, under the Regulations Concerning the Hygiene Supervision over Cosmetics, only 14 new ingredients were approved for filing from 2004 to 1 May 2021. See Table 3 for details:
Table 3 New Cosmetic Ingredients Approved for Filing from 2004 to May 1, 2021
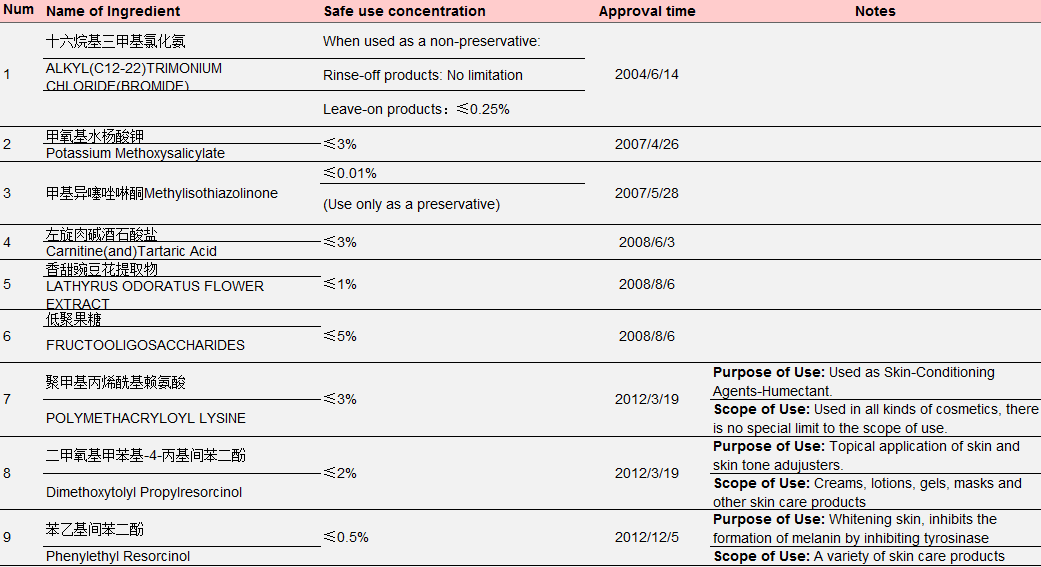
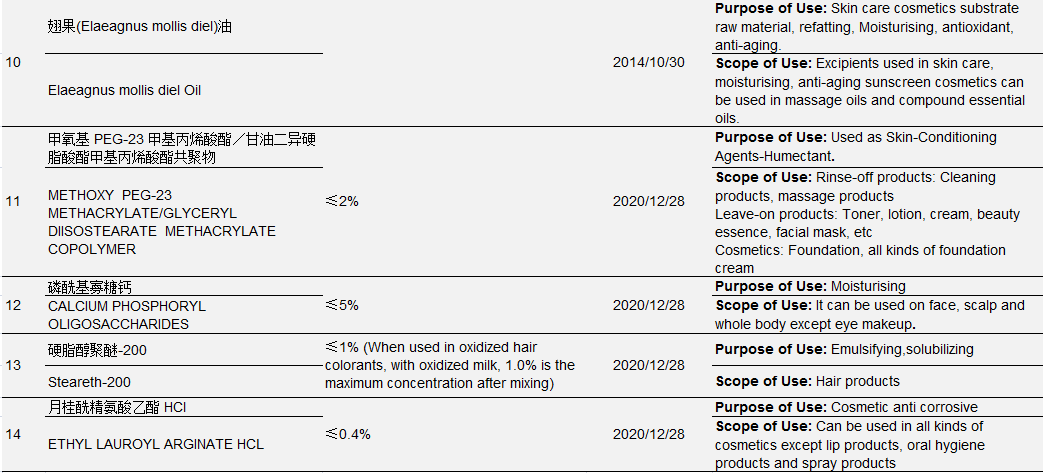
Data Source: Official Website of the Competent Department
Ingredient No.1-No.6 in the above table were approved by the former Ministry of Health, No. 7- No. 10 were approved by the former China Food and Drug Administration (CFDA), No. 11-No. 14 were approved by NMPA. The approval process also witnessed restructuring of national institutions: In September 2008, the health administrative licensing for cosmetics was passed over from the Ministry of Health to the State Food and Drug Administration; On March 22, 2013, the State Food and Drug Administration (SFDA) was renamed China Food and Drug Administration (known as CFDA); On April 10, 2018, CFDA was replaced by NMPA as a result of institutional restructuring.
If you would like to know more about the new cosmetic ingredients approved for filing, please refer to our Chinese Cosmetic Ingredient Regulatory Database (ChinaCosIng)
If you have any needs or questions, please contact us at service@cirs-group.com.

