REACH requires all companies manufacturing or placing a substance on the EU market in quantities greater than one tonne per year (1t/y) to register that substance with the European Chemicals Agency (ECHA). For legal reasons, only companies with a legal entity in Europe are allowed to submit a registration under REACH. However, non-EU companies can submit a REACH registration by appointing an Only Representative (OR) to register on their behalf, in which case their importers will be regarded as downstream users and do not need to do registrations.
Related Link
REACH Registration Compliance Guide: CIRS Helps You Meet Dossier Update Requirements!
The Scope of a REACH Registration
- A substance manufactured or imported above 1t/y on its own or in preparations; (Note: some substances are exempted).
Substances in articles if present above 1 t/y and intended for release (for example, ink in a pen);
Monomer substances if present at a concentration above 2% in a polymer (for polymers, monomers shall be registered);
Intermediates – reduced requirements and lower costs;
Substances subject to Product and Process Oriented Research and Development (PPORD) – are exempted from registration for five years. However, a PPORD notification must be submitted.
Who Shall Submit REACH Registrations
Manufacturers/Importers in EU;
REACH only representative appointed by non-EU manufacturers;
The Deadline for REACH Registration
Substances can be categorized into two groups under REACH:
- phase-in substances and
- none phase-in substances.
Each group has a different REACH registration deadline.
Phase-in substances (existing substances) enjoy the benefits of extended registration deadlines if pre-registered before December 2008. The principle is that the higher the tonnage, the earlier the registration deadline.
None phase-in substances (new substances not covered by the definition of a phase-in substance) need to be registered immediately before being placed in the EU market.
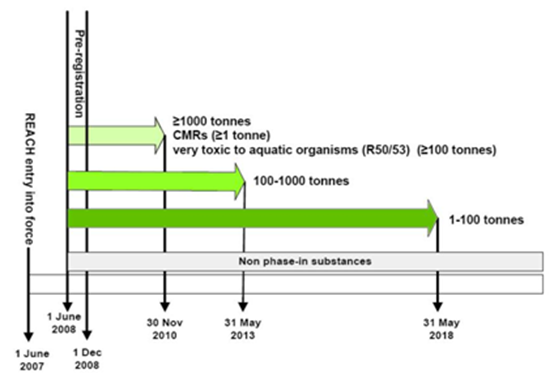
Definition of a Phase-in Substance
A substance that meets at least one of the following criteria:
It is listed in the European Inventory of Existing Commercial Chemical Substances (EINECS).
It was manufactured in the Community, or in the countries accepted to the European Union on January 1, 1995, or on May 1, 2004, but not placed on the market by the manufacturer or importer, at least once in the 15 years before the entry into force of this Regulation, provided the manufacturer or importer has documentary evidence of this.
It was placed on the market in the Community, or in the countries accepted to the European Union on January 1, 1995, or on May 1, 2004, before entry into force of this Regulation by the manufacturer or importer and was considered as having notified in accordance with the first indent of Article 8(1) of Directive 67/548/EEC but does not meet the definition of a polymer as set out in this Regulation, provided the manufacturer or importer has documentary evidence of this.
Phase-in substances that missed pre-registration cannot enjoy the benefits of the extended registration deadline and must be registered immediately.
Only Representative (OR)
Non-EU companies may submit registration by appointing an EU-based OR to register on their behalf. An OR must be an EU-based legal entity that has a sufficient background in the practical handling of substances and the information related to them required by the REACH regulation. Importers will be exempt from REACH registration if their non-EU suppliers have registered. However, importers need to confirm with their suppliers' OR that they are included in the importer list and that their tonnage and uses are covered by the OR.
More about REACH Only Representative
About the CIRS Group
The Chemical Inspection and Regulation Service (CIRS) Group is a leading product safety and chemical management consulting firm providing valued product regulatory compliance service, tailored solutions, and original information to help clients gain a competitive advantage by reducing business risks associated with regulatory affairs and removing barriers to entry. We have provided cost-effective regulatory support to over 3,000 companies while doing business in both the EU and China.
Since 2007, we have:
pre-registered over 10,000 substances;
registered over 2,000 substances;
served as the lead registrant (LR) for over 200 substances;
prepared over 5,000 REACH safety data sheets (SDSs) and classification, labelling and packaging (CLP) labels to date;
acted as the OR for over 3,000 non-EU companies;
served clients in more than 25 countries;
Our Services
- Only Representative (OR) Service;
- Lead Registrant;
- Joint Submission;
- Dossier Evaluation/Updating;
- Chemical Safety Report (CSR) Compilation;
- Testing Coordination/Supervision;
- Alternative methods (QSAR, Read-Across, In-vitro, Grouping, and more);
- SIEF Management Service;
- Development of Exposure Scenario (ES);
- REACH Technical Training Services.