KKDIK was published on June 23, 2017, by the Ministry of Environment and Urbanization of the Turkish Republic and formally came into effect on December 23, 2017. The regulation shares many similarities with EU REACH, thus it is also called Turkey REACH.
Scope of Registration
Substances manufactured in or imported into Turkey in quantities of one tonne or more per year;
Substances in mixtures manufactured in or imported into Turkey in quantities of one tonne or more per year;
Substances in articles manufactured in or imported into Turkey that are intended to be released under normal or reasonably foreseeable conditions of use and the quantity totals one tonne per year or more.
Who must Complete KKDIK Registration?
Manufacturers and importers of substances (including substances in mixtures) in Turkey;
Manufacturers and importers of articles (intended release) in Turkey;
Non-Turkish manufacturers of substances, mixtures, and articles must perform the registration obligations under KKDIK via an only representatives (OR) based in Turkey.
Timeline for Registration/Pre-registration
KKDIK Registration can be divided into two steps:
Step 1: Pre-registration
Step 2: Registration.
Details are as follows:
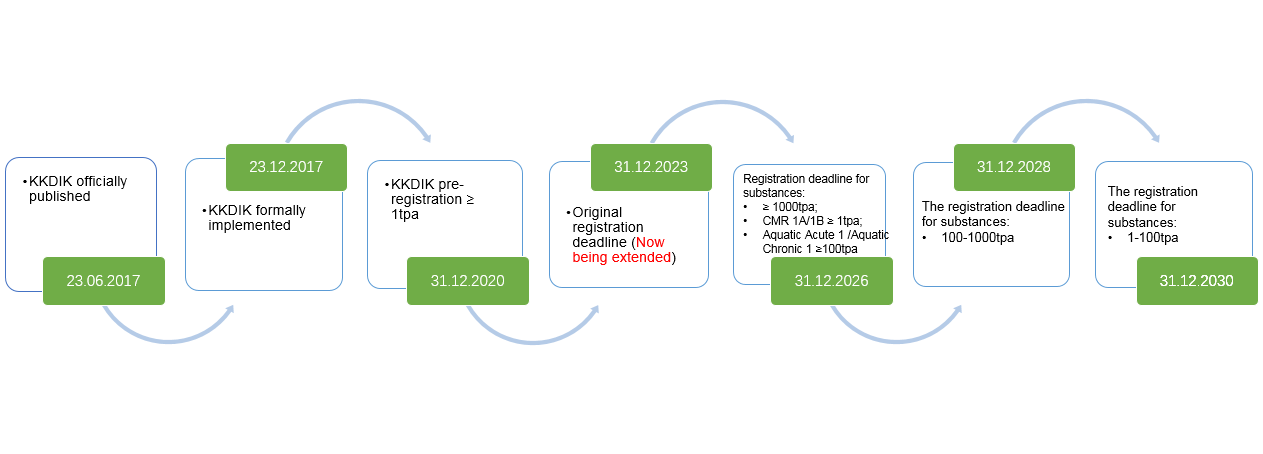
News Updates
On December 23, 2023, Turkey officially announced to extend the KKDIK registration deadlines to 2026, 2028 and 2030 depending on the tonnage band and hazard classification, namely:
(1) Substances meeting the following conditions should be registered before December 31, 2026:
- Substances manufactured or imported in their own or in a mixture or in goods in an annual amount of 1000 tonnes or more,
- Substances manufactured or imported in their own form or in a mixture or in goods in an annual amount of 100 tonnes or more, and that are within the Aquatic Acute 1 and Aquatic Chronic 1 (H400, H410) hazard categories according to the Regulation on the Classification, Labelling, and Packaging of Substances and Mixtures,
- Substances manufactured or imported in their own form or in a mixture or in goods in an annual amount of 1 tonne or more, and that are within the Carcinogenic, Mutagenic, and Reprotoxic Category 1A and 1B hazard categories according to the Regulation on the Classification, Labelling, and Packaging of Substances and Mixtures.
(2) For substances manufactured or imported in their own form or in a mixture or in goods in an annual amount of 100 tonnes or more, the deadline for registration is December 31, 2028;
(3) For substances manufactured or imported in their own form or in a mixture or in goods in an annual amount of 1 tonne or more, the deadline for registration is December 31, 2030.
Information Required for Pre-registration
Substance information (such as substance name, CAS No., EC No., and substance structure)
Registration Type
Registration type | Tonnage band | Data requirements |
|---|---|---|
On-site isolated intermediates under strictly controlled conditions | >= one tonne per year (1t/y) | Existing data only |
Transported isolated intermediates under strictly controlled conditions | >=1t/y | Existing data only |
>=1000t/y | KKDIK Annex VII | |
Registration of regular substances
| >=1t/y | A) 1-10t/y standard registration, all data specified in Annex VII; B) 1-10t/y, The information on physicochemical properties specified in Annex VII, for substances that do not meet either of the criteria specified in Annex 3; |
>=10Tt/y | KKDIK Annex VII+VIII | |
>=100t/y | KKDIK Annex VII+VIII+IX | |
>=1000t/y | KKDIK Annex VII+VIII+IX+X | |
Note: for >=10t/y regular registration, a chemical safety report (CSR) is also needed. | ||
1. Intermediates can be divided into three categories: Non-isolated intermediates, on-site isolated intermediates, and transported isolated intermediates.
Non-isolated intermediates: an intermediate that during synthesis is not intentionally removed (except for sampling) from the equipment in which the synthesis takes place.
On-site isolated intermediate: an intermediate not meeting the criteria of a non-isolated intermediate and where the manufacture of the intermediate and the synthesis of (an)other substance(s) from that intermediate take place on the same site, operated by one or more legal entities;
Transported isolated intermediates: an intermediate not meeting the criteria of a non-isolated intermediate and transported between or supplied to other sites;
Polymers: Similar to EU REACH, polymers are exempted from registration under KKDIK. However, monomers or other reactants of polymers shall also be registered when they meet certain conditions.
Definition of polymer: a substance consisting of molecules characterized by the sequence of one or more types of monomer units, distributed over a range of molecular weights wherein differences in the molecular weight are primarily attributable to differences in the number of monomer units and comprising the following:
1) A simple weight majority of molecules containing at least three monomer units which are covalently bound to at least one other monomer unit or other reactants; and
2) Less than a simple weight majority of molecules of the same molecular weight.
Monomer: a substance that is capable of forming covalent bonds with a sequence of additional like or unlike molecules under the conditions of the relevant polymer-forming reaction used for the particular process;
Polymers can be exempt from registration. But if their monomers and other reactants fulfill the following conditions, registration is required:
a) The polymer consists of 2% weight by weight (w/w) or more of such monomer substance(s) or other substance(s) in the form of monomeric units and chemically bound substance(s);
b) The total quantity of such monomer substance(s) or other substance(s) makes up one tonne or more per year.
Our Services
KKDIK Regulatory Compliance Consulting and Training;
KKDIK OR Service;
KKDIK Pre-registration;
KKDIK Registration;
Turkey CLP Regulation Regulatory Compliance Consulting and Training;
Turkey CLP Regulation OR Service;
C&L Notification under Turkey CLP Regulation;
Turkish SDS and Labeling Preparation;