On December 5, 2017, CFDA issued a notice on regulating cosmetics registration and filing application (Decree No.195,2017), in order to ensure that the company information on the new version of Cosmetics Production License and recorded products is consistent. For approved domestic special use cosmetics, the application for the change of company info is not required when the actual production place has no change, but should apply for the change when applying for license renewal or the change of other matters. For recorded domestic non-special use cosmetics, in the event that a new version of the Cosmetics Production License causes a change in the company ID for record keeping and the info of recorded cosmetics, the company shall log into the domestic non-special use cosmetics filing system within 6 months from the date of issue of this announcement, and voluntarily change the relevant filing information of the products according to the requirements of the system.
Policies regarding the management of
domestic cosmetic registration and filing have been frequently issued by CFDA
in order to ensure consumers' health and benefits. Based on years of experience
in cosmetic regulatory service, the CIRS cosmetic team has deeply analyzed the
current situation of domestic cosmetics industry in China.
Relevant regulatory policies
Since 2013, the CFDA has gradually standardized the production, registration and record-keeping of domestic cosmetics to strengthen the supervision, and has issued a series of regulatory documents.
- Notice on adjustment of the management of cosmetics registrations and filing (Decree No.10,2013)
- Notice on further work of the current cosmetic production license (CFDA Decree No.213,2013)
- Letter on further clarifying the implementation of the registration and filing of cosmetics (CFDA memo [2014] No. 70)
- Notice on the matters relating to the cosmetic production license (Decree No.265,2015)
- Notice on enabling the cosmetics production license uniformly (Decree No.12,2017)
- Notice on standardizing
the registration and filing of cosmetics (Decree No.195,2017)
Current status of domestic non-special use cosmetics
The monthly record of domestic non-special use cosmetics is counted in ten thousands and the amount of record from Guangdong province takes more than 70% of the total record amount. (the record amount of domestic non-special use cosmetics mentioned in this paper is counted in the record number). Shanghai, Zhejiang and Beijing ranked second, third and fourth with about 12%, 5% and 4% of total filings respectively. Figure 1 shows the number of application of domestic non-special use cosmetics from each province, the total of which has reached 200,000. The total number of records handled by local bureaus will exceed 300,000 if taking contract manufacturing products into account as well.
In 2015 and 2016, the numbers of recorded domestic non-special use cosmetics were 217,254 and 202,803 respectively. In 2017, the total number has reached 272,223 as of November 30. Due to the implementation of "integrating two certificates into one license" for Cosmetics Production License, domestic enterprises were in a transition period of license change, during which the number of recorded products has been affected.
On November 30, the record number of domestic non-special use cosmetics in Guangdong province was "粤G妆网备字2017172138". In 2015 and 2016, the record number of domestic non-special use cosmetics ended up with "粤G妆网备字2015136731” and “粤G妆网备字2016128659" respectively. The annual recorded domestic non-special use cosmetics in Shanghai exceeded 20,000 and 30,000 by the end of November 2017. The annual recorded number in Zhejiang is around 12,000. From figure 1, it is not difficult to find that the annual record number of Inner Mongolia, Guizhou, Tibet and Qinghai is less than 200, but it is also in the trend of growth.

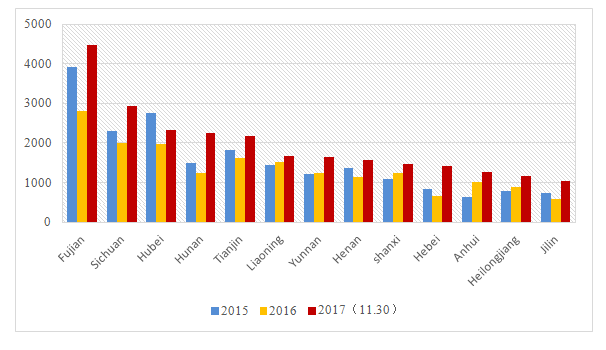
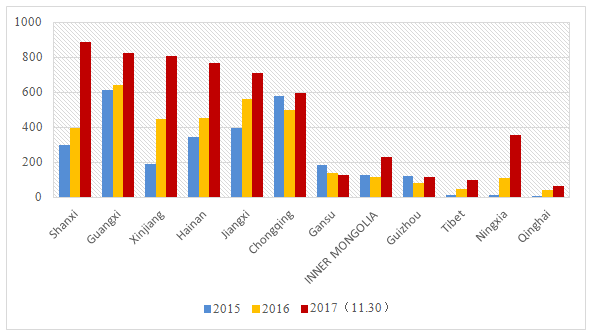
Figure 1, annual record number of domestic non-special use cosmetics from each province*
* Only counted from the record number of each province
Analysis of annual registration number of domestic special use cosmetics
In recent years, the amount of domestic special use cosmetics registration has a relatively large fluctuation. According to the Notice on Adjustment of the Management of Cosmetics Registrations and Filing issued in December 2013 (Decree No.10,2013), whitening cosmetics were managed as spots removal cosmetics. As a result, enterprises are in a transition period for the application for license of whitening cosmetics in 2014, basically. In the meantime, "the NOTICE" also made it clear that the recorded whitening products have not been re-applied and obtained the license as special use cosmetics, since 30th of June, 2015, would not be manufactured or imported. Therefore, the amount of application of domestic spots removal cosmetics increased dramatically in 2015, of which more than 2000 units were approved. In 2013 and 2014, the approval of spots removal cosmetics was only about 400. The implementation of "integrating two certificates into one license" for Cosmetics Production License on January 1, 2016 and implementation of Safety and Technical Standard for Cosmetics on December 1, 2016 had a great influence on the registration of domestic special use cosmetics.
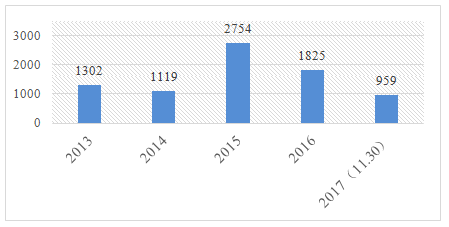
Figure 2. Annual amount of registration of domestic special use cosmetics
Enterprises acquiring cosmetics production license
The amount of registration and record
keeping of domestic products in each province depends largely on the number of
local manufacturers and brand owners. Since the official implementation of
"integrating two certificates into one license" for Cosmetics Production
License, most of the production manufacturers have made some modifications of
the production. From January 1, 2017, Cosmetics Production License was enabled
uniformly. The National Industrial Production License and Cosmetics
Manufacturer Hygiene License owned by the manufacturers became invalid
automatically. So far, there were 4,049 licensed enterprises, in which 2224
were from Guangdong province, accounting for about 55%. The number of licensed
enterprises in Zhejiang, Jiangsu and Shanghai also exceeded 200.
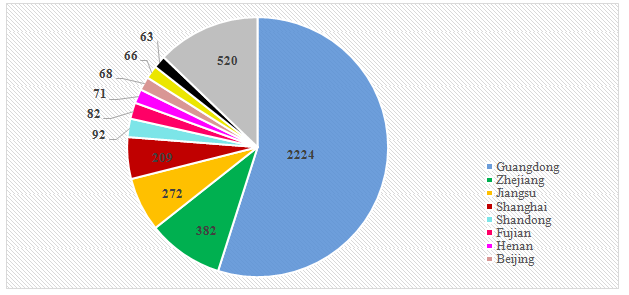
Figure 3. Areal distribution of enterprises having cosmetics production license
Domestic cosmetics are now in solid development, local and small size brands are emerging and local cosmetics giants are developing new products according to market demand. Meanwhile, in consideration of animal protection and production cost, many foreign brands also put some products into China for production. If local cosmetics manufacturers want to secure their seats in the local market, the compliance of their products should not be ignored. For the registration of domestic special use cosmetics and record keeping of domestic non-special use cosmetics, more information is accessible through CIRS's relevant documents.
If you have any other needs or questions, please contact us at service@cirs-group.com.

