On November 9, 2023, the European Union Scientific Committee on Consumer Safety (SCCS) published the preliminary opinion(SCCS/1658/23) on Hexyl Salicylate (CAS/EC No. 6259-76-3/228-408-6). The deadline for comments was set for 12 January 2024.
The SCCS concludes the following:
1. In light of the data provided and taking under consideration the CMR Cat.2 classification (to be introduced in Annex VI to Reg. 1272/2008), does the SCCS consider Hexyl Salicylate safe when used up to the maximum concentrations provided in the dossier?
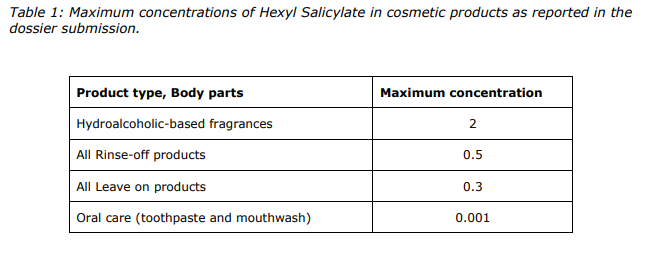
Based on the assessment of data provided and taking into consideration the concerns related to potential endocrine disrupting properties, the SCCS considers Hexyl Salicylate safe when used up to the maximum concentrations as provided in Table 1 of this Opinion.
2. Does the SCCS have any further scientific concerns with regard to the use of Hexyl Salicylate in cosmetic products?
The SCCS mandates do not address environmental aspects. Therefore, this assessment did not cover the safety of Hexyl Salicylate for the environment.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

