According to the Supervision and Administration Regulations (CSAR), the efficacy claims of cosmetics should have a sufficient scientific basis. Cosmetics registrant and filer should publish a summary of the literature, research data, or product efficacy evaluation information on the efficacy claims to the NMPA-designated website for public supervision.
Cosmetic registrant and filer should be responsible for the scientificity, authenticity, reliability, and traceability of the submitted summary of the basis for the efficacy claims.
Exemption of Efficacy Evaluation
Sensory recognition by way of the visual or olfactory system
-ex. cleaning, makeup removal, beauty and modification, fragrance, refreshing, hair dye, hair perm, hair color care, depilation, deodorization, and auxiliary shaving. etc.
Physical effect by the way of covering, adhesion, friction
-ex. whitening, exfoliator, deep pore cleanser (labeled as having physical effect only)
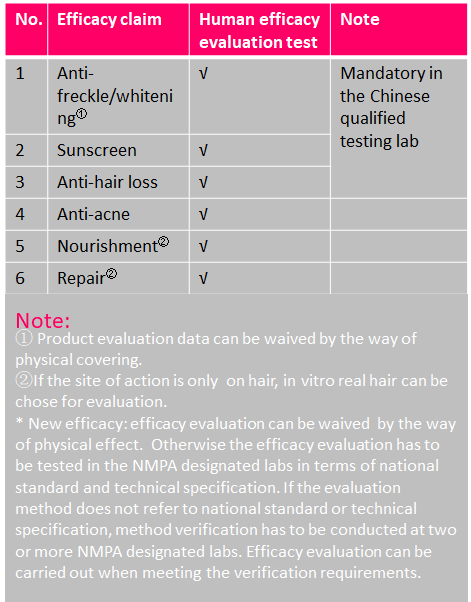
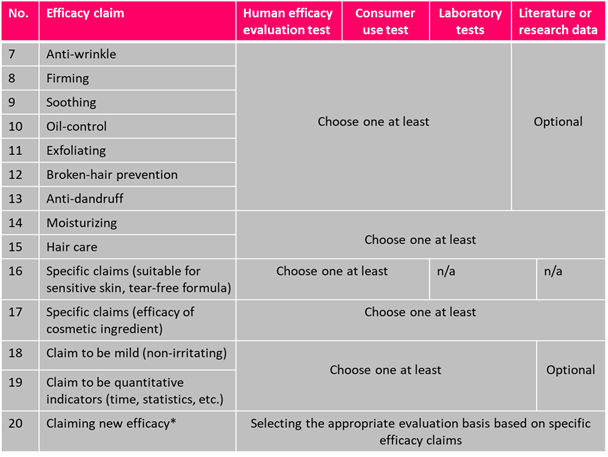
Efficacy Evaluation Requirements of Cosmetics
Transitional Solutions
Cosmetics efficacy claims should be evaluated in terms of the Specification for the Evaluation of Cosmetic Efficacy Claims and a summary of the basis for product efficacy claims must be uploaded to the NMPA designated website | New cosmetic registration and filing | Cosmetics that have been | Cosmetics that have been |
|---|---|---|---|
Since 2022 | Before May 1, 2023 | Before May 1, 2022 |
Our Services
- Clinical Efficacy Evaluation
- In-vitro Efficacy Evaluation
- In-vitro Safety Evaluation
- Cosmetic Testing for China
Further Information