Recently, European Food Safety Authority (EFSA) published the updated list of QPS-recommended microorganisms, in which six new taxonomic units (TUs) received the qualified presumption of safety (QPS) status and one existing microorganism was withdrawn.
In China, the QPS list can be commonly found in the approval information of microorganisms from the announcement interpretation of “Three New Foods” issued by the national health commission (NHC). People may be confused about what the QPS list is, and how it works in the compliance of microorganisms in China. Therefore we will cover the two questions mentioned above in three parts:
- the introduction of QPS;
- the content of the update; and
- its significance in the declaration of “Three New Foods”.
QPS: the microorganisms safety assessment system of the European Union
Qualified Presumption of Safety (QPS) is a management system proposed by European Union in 2017, and used for assessing the safety of microorganisms used in feed and food products before they are authorized for use in the European market. The Biological Hazards (BIOHAZ) panel is responsible for reviewing the relevant scientific literature on the safety of all microorganisms on the QPS list and assessing new microorganisms notified to EFSA. The certified strains will be included in QPS list which is updated every six months, and whose scientific opinion that provides more detail on the assessment is published every three years.
- Assessment Object: the microorganisms added to feed additives, food additives, food enzymes, food flavorings, novel food, and plant protection products (“regulated products”). Enterprises using microorganisms out of the list for food production must apply for a safety assessment. QPS assesses microorganisms with a long history of consumption while microorganisms that do not have traditional eating habits need to be approved by the European Union as novel foods.
- Assessment Content: four main contents are included: taxonomic status, knowledge system, pathogenicity, and end-use application. Most of the strains on this list can be used for a variety of purposes, but some are limited. By adding “restrictions” to the QPS list and authorizing their usage, the safety of authorized strains can be ensured.
Update of the list of qualified presumption of safety (QPS)
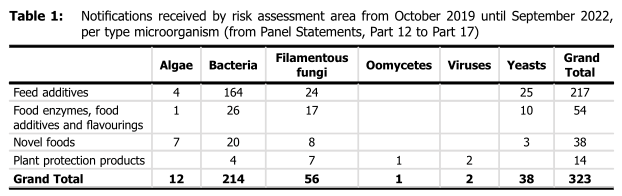
- Update notifications: between October 2019 and September 2022, 323 notifications were received and from these:
- 217 were for microorganisms used for the production of feed additives;
- 54 for food enzymes, food additives and flavorings;
- 14 as plant protection products (PPPs); and
- 38 for novel foods:
- Withdrawal of Bacillus velezensis
The qualification ‘absence of aminoglycoside production ability’ was withdrawn for Bacillus velezensis based on recent information that did not support possible aminoglycoside production, thereby contradicting the original data on which the qualification was based (EFSA BIOHAZ Panel, 2023).
- Six new TUs received the QPS status
As stated in the scientific opinion, six new TUs received the QPS status between October 2019 and September 2022:
Category | Latin name | Qualifications for QPS status |
|---|---|---|
Algae | Haematococcus lacustris (synonym Haematococcus pluvialis) | for production purposes only |
Bacteria | Bacillus circulans | for production purposes only; absence of cytotoxic activity |
Bacillus paralicheniformis | absence of toxigenic activity; absence of bacitracin production ability | |
Geobacillus thermodenitrificans | absence of toxigenic activity | |
Lactiplantibacillus argentoratensis (previously Lactobacillus plantarum subs. Argentoratensis) | absence of safety concerns; (its former taxonomic position as a sub species of L. plantarum had the QPS status) | |
Yeasts | Ogataea polymorpha | for production purposes only |
The relationship between QPS list and the compliance of microorganisms in China
China adopts a combination of a list system and a dynamic approval system for the management of microorganisms used in food, which mainly includes: List of strains that can be used in food; List of strains that can be used for infants and children; List of probiotics that can be used in health food, and strains that are used in food production and processing traditionally. For strains not listed above, an application system has been implemented.
When conducting an application for a new strain in China, enterprises are required to provide sufficient safety data, such as strain toxicity, drug resistance evaluation, history of consumption, adverse effect reports, clinical research data, dietary exposure data, and international approval, among which the QPS list published by European Union can not only serve as a support basis for approval but also a reference to enterprises because of its scientific and comprehensive in the literature review.
For example, two new food raw materials, Lactobacillus kefiranofaciens subsp. kefiranofaciens and Bifidobacterium longum subsp. longum BB536, approved in 2021 and 2022, have been included in the list of recommended biological agents in the QPS list.
Summary
Last year, the NHC issued an update notice of the List of strains that can be used in food; List of strains that can be used for infants and children (No. 4 of 2022), which made supplements of the two lists and adjusted the classification of listed strains. It means that the management of microorganisms used in food in China has always been in line with international standards. In addition, since the further researches on strains used for food production develop constantly, whose function in the field of intestinal health has attracted great attention, more and more probiotics and postbiotics products have emerged (Click here to view more details: How Do You Clearly Distinguish Between Probiotics, Prebiotics, Synbiotics and Postbiotics?).
As mentioned above, microorganisms out of the approval list must not be used in food production in China unless to be approved as new food raw materials. The QPS system of the European Union can serve as a reliable basis for safety assessment in China.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
References
[1] https://www.efsa.europa.eu/en/topics/topic/qualified-presumption-safety-qps
[2] https://www.efsa.europa.eu/en/efsajournal/pub/7747
[3] 李凤琴.食品微生物菌种安全性评估研究进展[J].中国食品卫生杂志,2018,30(06):667-672.
[4] 葛媛媛,姚粟,赵婷,程池.欧洲食品安全局食品用微生物菌种的管理概述[J].食品与发酵工业,2014,40(06):142-146+151.
[5] 我国食品用菌种安全性管理现状及国内外管理方式对比研究,食品科学技术学报,2020.
[6] 中国食品科学技术学会益生菌分会.后生元的研究现状及产业应用[J].中国食品学报,2022,22(08):416-426.

