1. Background and Definitions
GRAS (Generally Recognized as Safe) is a critical category under US food regulations, applicable to the safety assessment of both human food and animal food ingredients. If a substance is generally recognized as safe (GRAS) under its intended conditions of use, it falls outside the definition of a "food additive" and is exempt from the premarket approval requirements of the Federal Food, Drug, and Cosmetic Act (FD&C Act).
The FDA regulates GRAS ingredients in animal food through the GRAS Notification Program. Food companies and manufacturers can either submit a GRAS Notice (GRN) to the FDA to receive a “No Questions” letter or independently draw the GRAS conclusion based on the opinions of the panel group.
2. Submission Trends
Overall Submission Status
As of March 28, 2025, the FDA has publicly disclosed 75 Animal Food GRAS Notices on its official website. Among these, 37 GRNs received the “No questions” letters from the FDA, six GRNs failed to provide a basis for a GRAS determination, and four GRNs remain pending. The notifiers of the remaining 28 GRNs asked the FDA to cease evaluation.
Annual Submission Trends
Since the first Animal Food GRAS Notice was submitted in 2010, the number of filings has steadily increased. Notably, 2021 saw the highest GRN submissions (12), with five receiving the FDA’s “No questions” letters, and 2024 recorded eight GRN submissions, while three of them received “No questions” letters.
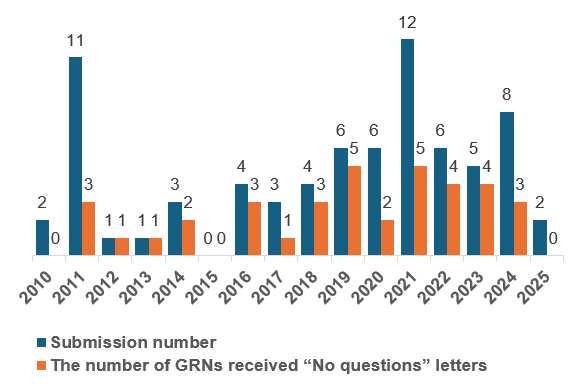
Figure 1. Annual Submissions of Animal Food GRAS Notices
Comparison with Human Food GRAS
The rate of Animal Food GRAS Notices receiving the FDA’s “No questions” letters is below 50%, which is significantly lower than that for Human Food GRAS Notices (~80%). Additionally, the total number of Animal Food GRN submissions (75) is far fewer than Human Food GRAS (1,234 as of May 2025). Nevertheless, the program timelines for both categories are similar, typically six months, and can be extended to nine months if necessary.
3. Animal Food GRAS Substance Categories
Among the 37 Animal Food GRAS Notices that received the FDA’s “No questions” letters, as many as 15 substances were produced involving genetically modified (GM) processes, including enzyme preparations and amino acids. Alternative proteins and transgenic modification technologies are becoming popular fields for Animal Food GRAS.
Table 1. Substance Categories of Animal Food GRNs received “No Questions” letters
Substance category | The number of GRNs received “No questions” letters | Remark |
Enzyme preparations | 11 | All produced via genetically modified microorganisms |
Functional additives | 6 | Antifoaming agents, antioxidants, anticaking agents |
Amino acids | 6 | Four of them were produced via genetically modified microorganisms |
Protein sources | 5 | Microbial protein, animal protein |
Live microorganisms | 3 | – |
Other nutrients | 6 | Algal oil, glycans, saturated fatty acids, oxidized β-carotene |
GM-derived substances (counted separately) | 15 | Enzyme preparations, amino acids |
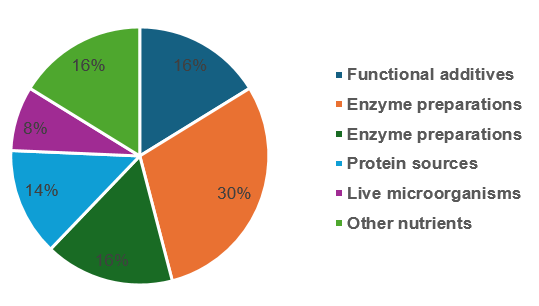
Figure 2. Substance Categories of Animal Food GRNs received “No Questions” letters
4. Target Species
Animal Food GRAS Notices typically target food-producing animals (for human consumption) or non-food-producing animals. Animal GRNs for food-producing species require additional safety data, including human dietary exposure and metabolic assessments. Currently, the majority of Animal GRNs receiveing the FDA’s “No questions” letters are intended for use for food-producing animals (27).
Table 2. Target Species of Animal Food GRNs received “No Questions” letters
Target animal category | The number of GRNs received “No questions” letters | Target species |
Food-producing animals | 27 | Poultry (chickens, ducks), livestock (pigs, cattle, sheep), aquaculture (fish, crustaceans) |
Non-food-producing animals | 6 | Pets (cats, dogs), horses |
All animals | 4 | – |
5. Notifiers (Submitting Companies)
Among the 75 Animal Food GRAS Notices, the leading companies with the most notices receiveing “No questions” letters are from the US. Other countries, including South Korea, the Netherlands, and Germany, also have related enterprises approved. So far, no Chinese enterprise has received “No questions” letters from the FDA for animal food. However, according to CIRS information, Chinese enterprises have already made an independent GRAS conclusion for animal food.
Table 3. Companies with Animal Food GRNs received “No Questions” letters
Notifier/ Company | Country | Number | Substance |
Agrivida, Inc. | US | 5 | Enzyme preparations |
CJ CheilJedang Corporation | South Korea | 4 | Amino acids |
DSM Nutritional Products DSM Innovation, Inc. | Netherlands | 4 | Enzymes, antioxidants, inactivated yeast |
Emerald Carolina Chemicals LLC | US | 3 | Antifoaming agents |
Native Microbials, Inc. | US | 3 | Live microorganisms |
BASF Enzymes LLC (BASF Corporation) | Germany | 3 | Enzyme preparations |
KnipBio, Inc. | US | 5 | Enzyme preparations |
Veramaris USA LLC | South Korea | 4 | Amino acids |
6. Conclusions
Submission Trends
Since 2020, the demand for Animal Food GRAS notices submitted to the FDA has grown, particularly in synthetic biology, microbial proteins, and GM-derived ingredients. Beyond US firms, global companies (e.g., South Korea, Netherlands, Germany) are increasingly participating in Animal Food GRN submissions.
Recommendations for Companies
Animal Food GRAS Notices are more challenging than Human Food GRAS, especially for food-producing species. It is recommended that enterprises prepare sufficient safety data and information in advance. At the same time, enterprises can choose professional food regulatory consultant agencies to ensure compliance, efficiency, and scientificity, avoiding timeline delays or related legal risks.
About Us
The Food Division of CIRS Group was established in 2012 and has a professional team specializing in US GRAS notices. The Food Division has extensive experience in various fields, covering GRAS, new food ingredients, new food additives, food contact materials, synthetic biology foods, EU Novel Foods, dietary supplements, and special dietary foods.
CIRS operates a fully-owned subsidiary in the US. By leveraging the expertise of the CIRS USA and the international team, it can provide enterprises with various US food services, including but not limited to:
- US FDA GRAS Notice;
- US FDA Dietary Supplement Structure/Function Claim Notification;
- US New Dietary Ingredient (NDI) Notification;
- US FDA Registration of Food Facilities; and
- US Food Label/Advertisement Information Review.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

