In the first half of 2022, State Administration for Market Regulation issued a total of 885 health food (dietary supplement) registration approvals, 134 of them are new health food products. CIRS conducted a detailed summary of these 134 new products, and analyzed them from the following points.
1. The Overall Situation of Health Food Registration Approvals Announced in the First Half of 2022
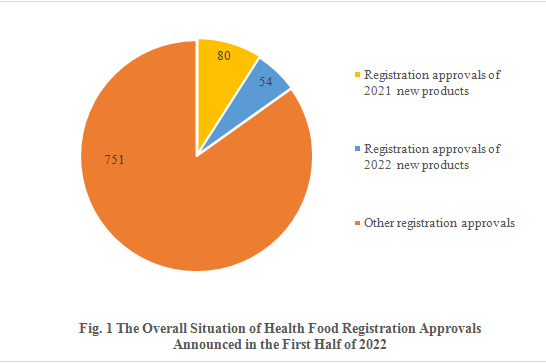
Among the 885 registration approvals, 134 are new products, accounting for 15.14% of the total. Of all the new products, 80 are approved in 2021 and 54 are approved in 2022. The other 751 registration approvals are not for new products, possibly renewal of registration, change of registration and transfer of technology registration.
2. The Number of Health Food Registration Approvals Announced in Every Month
As shown in Fig.2, registration approvals for 2021 new products are announced in January and February (shown in purple). The registration approvals for 2022 new products are not announced until April (shown in blue).
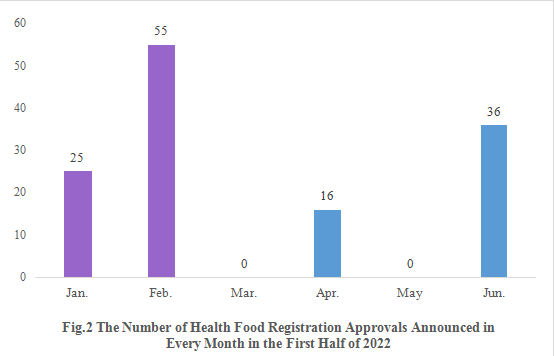
Note: 2 products are not included in the statistics since there is no release date for them.
3. The Number of Approved Products in Different Regions
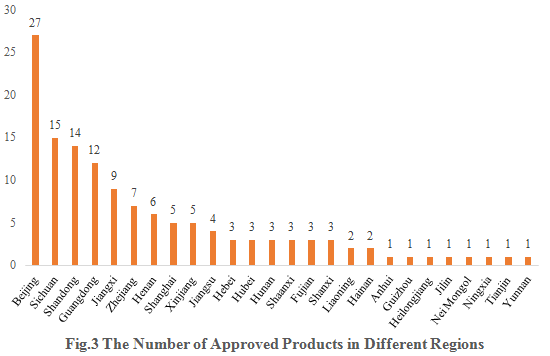
The 134 approved new products are from 26 provinces (municipalities and/or autonomous regions). Beijing, Sichuan and Shandong occupy the top three spots with the number of 27, 15 and 14 respectively, accounting for 20.15%, 11.19% and 10.45% of the total new products.
4. Enterprises Obtaining Health Food Registration Certificates
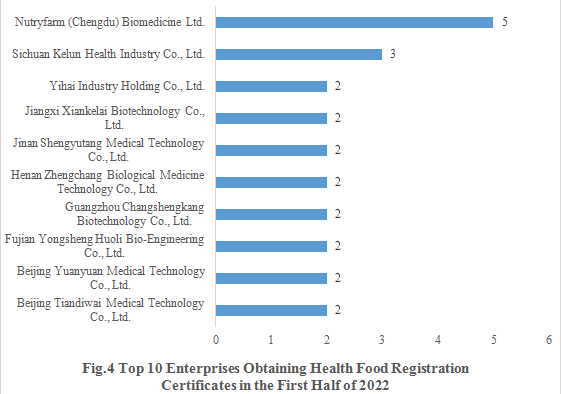
Nutryfarm (Chengdu) Biomedicine Ltd. obtained 5 health food registration certificates in the first half of 2022 (3 products for enhancing immune, 1 product for alleviating physical fatigue and 1 product for assisting blood lipids reduction, with the dosage forms including tablet, capsule and granule). The top ten enterprises obtaining new registration certificates in the first half of 2022 are shown in the Fig.4.
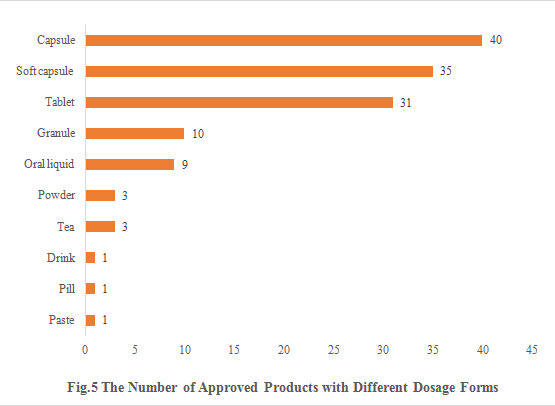
5. The Number of Approved Products with Different Dosage Forms
The dosage forms of approved new products include capsule, soft capsule, tablet, granule, oral liquid, powder, tea, drink, pill and paste. Among them, capsule products are the most, with the number of 40, accounting for 29.85%. The number of soft capsule products and tablet products are 35 and 31 respectively.
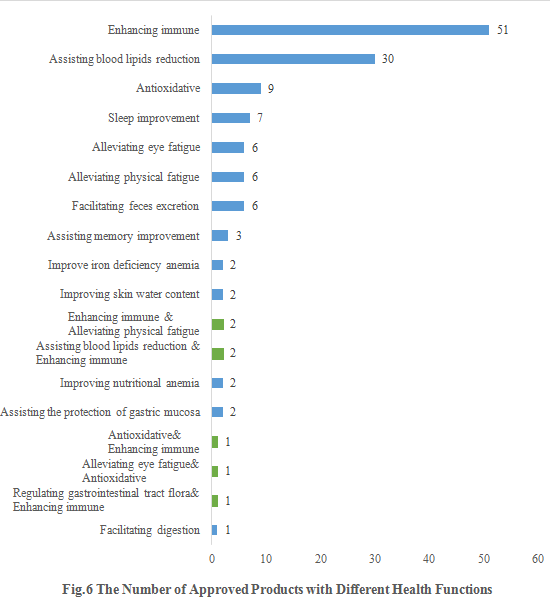
6. The Number of Approved Products with Different Health Functions
As shown in Fig.6, enhancing immune shares the largest number of approvals. The number of new products with the health function of enhancing immune is 51, accounting for 38.06% of the total new products. Besides, 7 products with the health function of sleep improvement are issued in the first half of 2022 (only 10 products with the same function were issued in the whole year of 2021).
Except for those new products with single health function, there are also 7 products approved with two health functions (shown in green). The health functions of the 7 products are enhancing immune & alleviating physical fatigue, assisting blood lipids reduction & enhancing immune, antioxidative & enhancing immune, alleviating eye fatigue & antioxidative and regulating gastrointestinal tract flora & enhancing immune.
7. The Main Functional (or Signature) Ingredients of the Products Corresponding to the Health Functions of Which Large Quantities of Products are Approved
Based on the information of products approved in the past two years, CIRS summarized the information of main functional (or signature) ingredients of the products corresponding to the health functions of which large quantities of products are approved.
Table.1 The Main Functional (or Signature) Ingredients of the Products Corresponding to the Health Functions of Which Large Quantities of Products are Approved
Health Function | Main Function (or Signature) Ingredients |
Enhancing immune | total saponins, crude polysaccharide, lycopene, procyanidine, total flavonoids, adenosine, 10-hydroxy-α-decenoic acid, total triterpenes, puerarin, amino acid, peptide, Vitamin E, Vitamin C |
Assisting blood lipids reduction | α-linolenic acid, phosphatidylcholine, lovastatin, total saponins, total flavonoids, linoleic acid, phytosterol, DHA, EPA |
Antioxidative | procyanidine, lycopene, β-carotene, Vitamin C, Vitamin E, SOD, taurine |
Sleep improvement | total saponins, crude polysaccharide, jujube seed saponin, total flavonoids, triterpenes, schisandrin, gastrodin, icariin |
8. CIRS Comments
The number of registered health food new products has dropped significantly because of the negative influence of the COVID-19 as well as the lack of the basic regulations. Nowadays, people pay more attention to their own health, and the audience of health food tends to be younger gradually. It is expected that the evaluation methods for health functions can be issued as soon as possible, so that the application of health food registration can be promoted smoothly and the health food market will become vibrant again.
Data Source: Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.
Note: There may be a lag in the data release of the Special Food Information Query Platform. The data in this article is only for reference, and the actual situation is subject to the official announcement.

