Recently, CIRS Group has assisted a leading domestic enterprise in the pharmaceutical ingredients and fragrances industry in successfully completing the submission of its first registration dossier as the lead registrant (LR) under KKDIK regulation and obtaining the registration number. The success of KKDIK (also known as Turkey REACH) registration reflects the technical strength of CIRS Group and its Turkish partners.
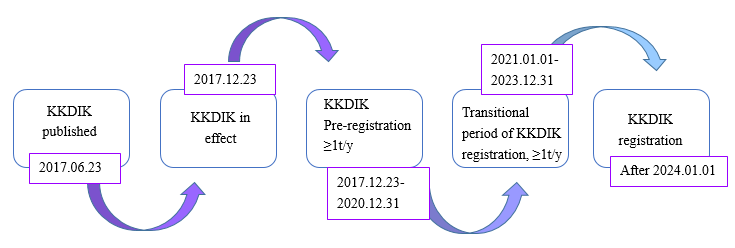
The KKDIK regulation, known as the Registration, Evaluation, Authorization and Registration of Chemicals in Turkish, was republished by the Ministry of Environment and Urbanization (MoEU) of the Republic of Turkey on June 23, 2017, and came into effect on December 23, 2017. Since then, manufacturers/importers are required to process pre-registrations under KKDIK within the deadline for substances exceeding ≥one tonne per year (1t/y) prior to manufacture/import.
The previous deadline for pre-registration was set on December 31, 2020. However, Turkish officials made adjustments. Currently, pre-registration is still available and will continue until December 31, 2023.
After January 1, 2024, the pre-registration number will be invalid. Suppliers outside of Turkey or importers in Turkey shall complete the registration of substances prior to import/export
KKIDK Regulation (Turkey REACH) is similar to EU REACH Regulation. Here are the procedures for KKDIK registration:
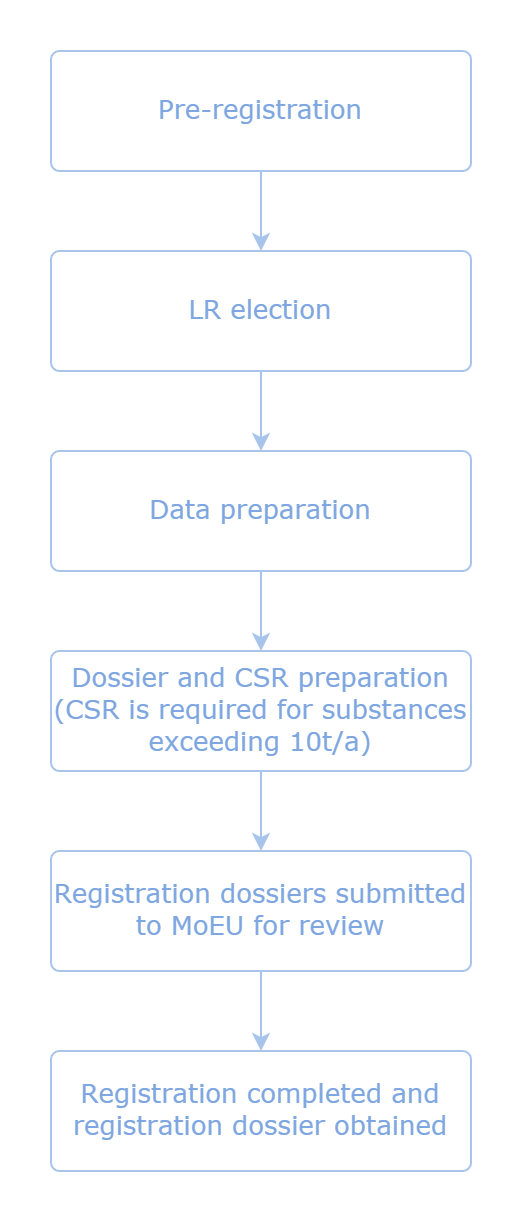
But unlike the EU REACH regulation, CSR shall be prepared by qualified professionals under Turkey REACH.
The Turkish company has previously entrusted CIRS Europe to serve as the only representative (OR) under EU REACH and become the lead registrant of the substance. As a result, there was complete data to cope with Turkey REACH registration, which made it possible for the company to carry out Turkey REACH registration without needing to carry out any additional tests for the substance. This means that it is available to handle registrations under multiple regulations with one set of data, thereby fully improving the utilization of data and saving additional data costs. In the next step, CIRS Group will help the company manage its SIEF, including data sharing, sales of LOA, and communication with the competent authorities to deal with the possible file evaluation.
Although the registration cycle under KKDIK is slightly slower than expected, it is still manageable for CIRS Group to deal with the KKDIK registration as planned. In the future, CIRS Group will continue to devote efforts to KKDIK Regulation research and try its best to make sure that the registration can be completed as planned as long as sufficient information is provided.
CIRS Group warmly reminds you that the deadline for KKDIK pre-registration is December 31, 2023. Enterprises that have completed pre-registration should confirm the LOA of substances and finish the full registration under KKDIK Regulation as soon as possible.






